Cytomegalovirus (CMV) infection is usually asymptomatic in immunocompetent patients. A mononucleosis-like syndrome may develop in some patients. Various organ involvements (eg: encephalitis, meningitis, retinitis, myocarditis, pneumonia, hepatitis, enterocolitis, neuritis), which rarely occur in immunocompetent patients, have also been reported. Cutaneous necrotizing vasculitis caused by CMV infection has been reported very rarely in the literature. Here, a case with a very rare clinical form of CMV infection, presenting with persistent fever and livedo reticularis on the extremities and cutaneous necrotizing vasculitis of the toes, is described, and the relevant literature is reviewed. This case report aims to highlight the possibility of CMV infection to be a cause of cutaneous necrotizing vasculitis.
Cytomegalovirus (CMV) infection is usually asymptomatic in immunocompetent patients.1 A mononucleosis-like syndrome may develop in some of the patients. Apart from that, various organ involvements (e.g.: encephalitis, meningitis, retinitis, myocarditis, pneumonia, hepatitis, enterocolitis, neuritis), which are rare in immunocompetent patients, have also been reported.2 Cutaneous necrotizing vasculitis caused by CMV has been reported very rarely.3–5 In this report, a very rare clinical form of CMV infection, presenting with persistent fever and livedo reticularis on extremities and cutaneous necrotizing vasculitis of toes, is described, and the literature regarding this case is reviewed.
Case presentationA 17 year-old female had been treated with amoxicillin-clavulunic acid, and clarithromycin for complaints of fever and cough. Her cough resolved within a month, but the fever persisted, and she started to present additional symptoms such as nocturnal sweating, livedo reticularis-like rash on hands and feet, and weight loss (12% of total body weight). There were no enlarged peripheral lymph nodes on physical examination. Laboratory investigation results during the first month of her illness were as follows: white blood cell count (WBC): 8,700/mm3, hemoglobin (Hgb): 9.7g/dL, hematocrit (Htc): 30%, platelets (PLT): 534,000/mm3, erythrocyte sedimentation rate (ESR): 70mm/hr, C-reactive protein (CRP): 44mg/L, alanine aminotransferase (ALT): 13 U/L, aspartate aminotransferase (AST): 12 U/L, creatinine kinase (CK): 19 U/L, total protein: 8.1mg/dL, albumin: 3.9mg/dl, ANA (antinuclear antibody): negative, anti-dsDNA (anti-double-stranded DNA): negative, urea: 30mg/dL, creatinine: 0.9mg/dl, urinary sediment: normal. Since this patient had been considered as a “fever of unknown origin” case and had the symptoms of fever, weight loss, and nocturnal sweating, thoracic and abdominal computed tomography (CT) scans were performed, along with bone marrow aspiration and biopsy to exclude lymphoma or any other malignancy. However, these exams revealed no abnormality. Suspecting the diagnosis of sustained mononucleosis syndrome, CMV serology was performed by ELISA. CMV IgM was 7.85 AU/mL (Abbott Architect i1000SR) and CMV IgG was 130.4 AU/mL (Abbott Architect i1000SR), and CMV IgG avidity was 4% (Abbott Architect i1000SR). CMV PCR was not initially tested. Human immunodeficiency virus (HIV), Epstein-Barr virus (EBV), toxoplasma and parvovirus serology were investigated for the differential diagnosis of mononucleosis syndrome, as well as hepatitis B virus (HBV) and hepatitis C virus (HCV) serology. Tests for EBV VCA IgM, toxoplasma IgM and IgG, anti-HIV, and parvovirus IgM, HBsAg, anti-HBc IgM and anti-HCV were all negative.
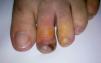
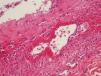
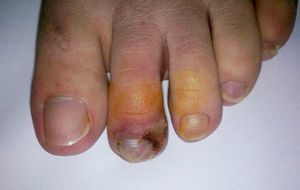
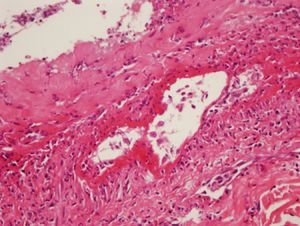
During the second month of her illness, fever, and bilateral livedo reticularis on her feet, more prominent on the left, continued. Repeated blood cultures remained sterile. No vegetations were detected on transthoracic echocardiography. No abnormality was detected on thoracic and abdominal CT. Arterial and venous Doppler ultrasonography of the upper and lower extremities and MRI (magnetic resonance imaging)-angiography of the aorta and its branches were normal. Markers for autoimmune diseases including ANA, anti-ds-DNA, RF (rheumatoid factor), anti-CCP (anti-cyclic citrullinated peptide), anti-LA (anti-SSB), anti-RO (anti-SSA), anti-ENA (anti-extractable nuclear antigen), Sm-antigen (Smith antigen), anti-ENA U1-RNP (U1- ribonucleoprotein), anti-cardiolipin IgM and IgG, MPO-ANCA (myeloperoxidase-anti-neutrophil cytoplasmic antibody), p-ANCA (perinuclear-ANCA), c-ANCA (cytoplasmic-ANCA) were all negative. CMV IgM was negative, but CMV IgG was > 250 AU/mL (Abbott Architect i1000SR) with low avidity (CMV IgG avidity: 4%, low avidity if less than 20%), PCR assay for CMV was negative. Other laboratory test results were as follows; WBC: 7.200/mm3, Hgb: 8.7 g/dL, Htc: 28.7%, PLT: 524,000/mm3, ESR: 76mm/hr, CRP: 39mg/L (negative if < 3 mg/L) and biochemical tests were within normal limits. As any infectious focus had not been detected, treatment with methylprednisolone 1mg/kg was begun. Although her fever resolved and ESR and CRP values decreased to normal levels, the tip of the second toe of her right foot had become necrotic (Fig. 1). The biopsy of this lesion revealed necrosis in the epidermis and dermis, necrotizing vasculitis, and occlusive vasculopathy of the small-sized vessels (Fig. 2). Inclusion bodies were not observed in the tissue specimen and CMV PCR was negative. Azathioprine 50mg twice a day and methylprednisolone 48mg was given to the patient. After the first month of immunosuppressive therapy, necrosis of the toes had begun to regress. She had no other systemic signs and symptoms, and she is still under follow-up at the outpatient clinic.
In recent years, some laboratory data regarding the association between viral infections and some autoimmune diseases have been obtained. Particularly, CMV-related conditions are remarkable. High CMV prevalence in systemic lupus erithematous patients, CMV antigen positivity in synovial fluid of rheumatoid arthritis patients, and presence of UL94-related apoptosis in vascular endothelium of systemic sclerosis cases are some examples.6–8
During the course of viral infections, viruses have been thought to cause vasculitis and vasculopathy either directly by replication in the vascular endothelium or indirectly by induction of autoimmunity.9 Some well-known examples are intra-synovial deposition of immune complexes against hepatitis B virus surface antigen or anti-HBsAg in polyarteritis nodosa, and hepatitis C virus-related cryoglobulinemic vasculitis.10,11 Despite conflicting results, studies describing parvovirus and herpes virus infections to cause temporal arteritis and giant-cell arteritis have been reported.12
CMV infection causes vascular endothelial damage either by causing direct cell injury and death or by means of immune-mediated injury via induction of autoimmunity (molecular mimicry). Other reported mechanisms of vascular injury are pro-coagulant activity increase and blockage of apoptosis in the endothelium. Favorable clinical response to antiviral and immunosuppresive treatments in the discussed cases points out to the possibility of CMV-related endothelial injury to occur by different mechanisms.9,13,14
Magro CM et al. have reported seven cases that had developed signs of cutaneous vasculitis after CMV infection. In these cases, although CMV infection had been confirmed serologically and virologically by molecular methods, specific inclusion bodies and virological evidence could not be obtained in tissue specimens. Good clinical response to antiviral therapy was reported for all but one patient.3 In the case described by Varani et al., sustained CMV mononucleosis was reported to be responsible for active endothelial injury and Wegener's granulomatosis by inducing autoimmune response. In the same case report, it was postulated that the accompanying polymicrobial urinary tract infection together with sustained CMV infection might have induced the vasculytic process in the kidneys. Nevertheless, this patient did not have any sign of localized infection supporting this hypothesis.15
Meyer et al. have reported that gancyclovir treatment alone, without intense immunosuppressive therapy, was successful in their patient with ANCA-positive systemic necrotizing vasculitis, which developed after systemic CMV infection.16 Besides, in the case reported by Nolan et al., treated with cyclophosphamide for Wegener's granulomatosis, although the systemic signs had resolved and the antibody titers had decreased, the foot ulcer had progressed. Gancyclovir therapy could only be started after the detection of CMV inclusion bodies in the biopsy specimen and good clinical outcome was reported.17
McCormick et al. demonstrated that digital vasculitis developing in a patient with rheumatoid arthritis was caused by immune-complexes against CMV antigen.18 Kuroda et al. have claimed the immune-complexes, produced against CMV antigen deposited in the vascular endothelium, to be responsible for acute hemorrhagic edema that developed during acute CMV infection in their patient, but they could not demonstrate the presence of CMV inclusions.4
In the current patient, presenting with persistent fever and livedo reticularis, radiological and immunological investigation did not reveal any signs of vascular pathology or connective tissue disease. Despite the very good response of fever and acute phase reactants to steroid therapy, the livedo reticularis, predominant in lower extremities, and the necrotic lesions that first developed in the right second toe and later in the toes of the left foot did not regress. Biopsy specimen showed the signs of necrotizing vasculitis, but there were no systemic signs and symptoms. Laboratory findings were completely normal. Except for autoimmune necrotizing vasculitis induced by CMV infection, no other rheumatologic pathology was detected. Fever persisting more than two months and the subsequent localized cutaneous vasculitis had been interpreted as complications of sustained CMV infection. Even though the serological tests (CMV IgM and IgG avidity) performed at the beginning and in the second month of the clinical course were consistent with acute CMV infection, CMV DNA by PCR assay was not demonstrated either in blood or in tissues. This is why antiviral therapy could not have been given to the patient.
ConclusionIn conclusion, during the course of CMV infection, signs of localized or systemic vasculitis may develop. In light of previously reported cases, it has been shown that some patients have benefited from antiviral therapy while others benefited from anti-inflammatory treatment. In order to decide which therapy would offer a better outcome for a given patient with CMV-related vasculitis, controlled studies including more patients are necessary.
Conflict of interestAll authors declare to have no conflict of interest.
The authors would like to thank Atay Uludokumacı, MD (Department of Pathology, Cerrahpasa Medical Faculty, Istanbul University) for his participation in the histopathologic examination of the biopsy specimen.