Disseminated strongyloidiasis is a disease with high mortality rate, especially in immunocompromised individuals. Paralytic ileus and intestinal malabsorption are frequent symptoms caused by this severe disease. As there are no licensed parenteral anthelmintic drugs for human use, off-label formulations are often used in the treatment of this disease. In this case report, the use of subcutaneous ivermectin is described as a successful therapy for this life-threatening infection.
Strongyloidiasis is caused by the nematode Strongyloides stercoralis, which infects 50 to 100 million people worldwide.1 This nematode uses the human body as a host and reproduces through autoinfection. The two aggressive forms of the disease are: hyperinfection syndrome or disseminated strongyloidiasis. In the first case, the infection occurs with a very heavy worm burden, while in the latter, the larvae penetrate the intestine wall and reach the bloodstream, causing bacteremia, meningitis, and septic shock.
The treatment of these severe forms of strongyloidiasis is still a matter of controversy for immunocompromised and critically ill patients who have reduced intestinal absorption of the oral formulations of antiparasitic drugs. In this case, the parenteral route is the most feasible and promising route for treatment since the drugs do not have to be absorbed by the gastrointestinal tract. In this report, a case of disseminated strongyloidiasis that was successfully cured with subcutaneous ivermectin (Ivomec® – Merial, Brazil – 10mg/mL solution) is described. However, it is important to stress that parenteral treatment with anthelmintics has not been approved for humans, and its safety is still questionable.
Case presentationA 56-year-old man from Brasília (Brazil), who was under ambulatory investigation for agranulocytosis and had been using corticosteroids for eight weeks (prednisone 80mg per day), came to this hospital with a history of intense asthenia. At admission, he was afebrile, hypotensive and, according to primary examination, presented with leukopenia and eosinophilia, which were treated with 2g IV cefepime q8h after collecting blood for cultures.
On the third day after admission, the patient's respiratory status deteriorated, and he was transferred to the intensive care unit. Despite non-invasive ventilation, he required intubation, mechanical ventilation, and vasopressor support by his fifth day in the hospital. Therefore, antibiotic escalation was prescribed to broaden the drug spectrum. Additionally, computed tomography scans of the thorax and abdomen were performed. The results of the former exam indicated bilateral areas of pulmonary condensation, and the latter was normal. Lastly, a pulmonary catheter was placed, which showed hemodynamic parameters indicating septic shock.
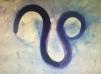
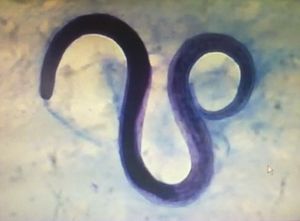
The patient's blood tests demonstrated a decreased hematocrit value, and therefore he underwent an esophagogastroduodenoscopy to rule out bleeding. In this exam, multiple vegetative lesions were observed, which were associated with erosions and diffuse enanthema in the duodenum. On the tenth day of hospitalization, infection with S. stercoralis was diagnosed based on the duodenal biopsy and the bronchoalveolar lavage fluid (Fig. 1), which were both performed simultaneously.
As soon as the nematode was detected, treatment was immediately initiated with ivermectin via the nasogastric tube, using 18mg daily, for two days, along with albendazol 400mg q12h. However, on the second day of treatment it became clear that this treatment was not as effective as expected and, with the consent of the patient's family, the treatment was continued with a veterinary formulation of subcutaneous ivermectin (Ivomec®; Merial, Brazil – 10mg/mL solution), and oral use of the drug was discontinued.
This therapy was very successful. On the third day of anthelmintic treatment, the patient was completely removed from vasopressor support. On the tenth day, mechanical ventilation was discontinued, and ten days thereafter, the patient was discharged from the ICU.
DiscussionThe patient's final diagnosis was disseminated strongyloidiasis with associated septic shock and acute respiratory distress syndrome. The key indicators leading to this diagnosis were the results from the bronchoalveolar lavage and the esophagogastroduodenoscopy. There have been similar symptoms related to this disease based on upper gastrointestinal endoscopy.2,3 However, these results are not specific to disseminated strongyloidiasis and are also associated with other pathological diseases.
Recently, reports4 have indicated that treatment of disseminated strongyloidiasis in immunocompromised patients with ivermectin is effective, although it is still a matter of controversy. It has been shown that enteral use of this medication could cause pharmacokinetic changes in case of paralytic ileus,5 jeopardizing its bioavailability and leading to lower concentrations of the drug than in its subcutaneous formulation (0.8 vs. 11.4-17.2 ng/mL).6 Conversely, it has also been argued that the oral route provides the appropriate plasma and cerebrospinal fluid concentration of the drug,7 contradicting the previously mentioned findings.
Furthermore, the pharmacokinetic properties of ivermectin may be modified in critically ill patients, because the drug is highly bound to human serum albumin.8 Systemic inflammation causes hypoalbuminemia, and therefore, both free drug concentration and therapeutic action are elevated.6 Nevertheless, a recent study, which was the first to document total and free levels of subcutaneous ivermectin,3 surprisingly found less than 1% of free ivermectin located in the plasma.3 A possible explanation for this finding is the strong binding between the drug and high alpha-1 acid glycoprotein concentration, which reduces the drug's distribution to tissues and contributes to poor therapeutic outcomes.3
Successful7 and unsuccessful3 treatments of disseminated strongyloidiasis were found. In a case report described by Rose et al.,8 the patient was treated exclusively with oral ivermectin, and this therapy was ineffective; the nematode was not eliminated, and the patient died. In other reports, however, enteral use of this drug successfully eradicated S. stercoralis, but the patient still died due to toxicity complications6,9 or due to the severity of the underlying disease.10,11 In the case described in the present article, favorable outcomes were documented, as no indication of larva were found in the stool or bronchoalveolar lavage fluid, and moreover, the patient survived and was discharged from the intensive care unit and the hospital.
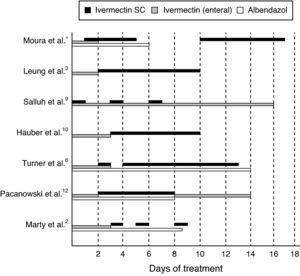
The dosage of subcutaneous ivermectin used was 15mg per day for the first four days (214μg/kg) and then, five days after discontinuation of the parenteral ivermectin, an additional seven-day therapy was initiated (20mg per day; 285μg/kg) because the patient presented with worsening neurological status and fever. Additionally, there was evidence indicating that the central nervous system was compromised. A few days later, the patient's symptomatology was diagnosed as herpetic encephalitis. The following table shows both successful and unsuccessful therapeutic procedures. However, because they were performed in different situations, they cannot be directly compared (Fig. 2).
Daily monitoring of the ivermectin concentration in the serum was essential, as the drug can be toxic to the central nervous system. The patient's persistent coma could have been confused with symptoms of toxicity even though the dose (285μg/Kg) was eight times lower than what can be tolerated by humans.12 It could also be due to herpetic encephalitis, but because the concentration of drug in the plasma could not be assessed, this hypothesis could not be confirmed.
With this study, the best treatment for disseminated strongyloidiasis cannot be definitely determined. Enteral use of ivermectin can result in therapeutic failure; in addition, in some situations, it does achieve adequate plasma levels. In this patient, parenteral use of this drug was essential for therapeutic success. Considering that data extrapolated from animal experiments are insufficient, it is extremely important that reports about the usage of parenteral ivermectin in humans are discussed, along with its viability and its toxicity, in order to continue to improve treatment for this devastating form of strongyloidiasis.
Conflict of interestAll authors declare to have no conflict of interest.
The authors would like to thank Paulo de Oliveira Martins Jr. from the Parasitology Laboratory of the Santa Luzia Hospital for his valuable cooperation.