Hepatitis B Surface Antigen (HBsAg) seroclearance is the highest treatment goal recommended by the current guidelines for hepatitis B. Levels of antibodies to HBsAg (anti-HBs) are strongly associated with HBsAg recurrence, but hepatitis B vaccination may increase the anti-HBs seroconversion rate and reduce recurrence. We conducted a retrospective clinical study to ascertain the effect of this vaccination on the seroconversion rate and levels of protective anti-HBs after HBsAg. In this retrospective study, we distributed a questionnaire through an online survey platform to collect information related to hepatitis B vaccination in patients with functional cure of hepatitis B with Interferon-α (IFNα) therapy. We enrolled 320 patients who achieved functional cure from IFNα therapy. Of these, 219 patients had received hepatitis B vaccination according to their personal preference and drug accessibility after HBsAg seroclearance, whereas the remaining 101 patients did not receive hepatitis B vaccination. The anti-HBs seroconversion rate of 78.1% in the vaccinated group was significantly greater than that in the unvaccinated group (41.6%) (p < 0.001). Stratified comparisons with anti-HBs of ≥ 100 IU/L and ≥ 300 IU/L showed that both proportions in the vaccinated group were greater than those in the unvaccinated group (71.2% vs. 32.7% and 56.2% vs. 17.8%, respectively, all p-values < 0.001). Logistic regression analysis showed that the odds ratio of vaccination was 4.427, which was the strongest influencing factor for anti-HBs, reaching 100 IU/L or higher. Hepatitis B vaccination in patients after HBsAg seroclearance not only increased the anti-HBs seroconversion rate but also significantly increased antibody levels, with good safety, indicating the clinical value of vaccine therapy for patients with functional cure.
Hepatitis B surface Antigen (HBsAg) seroclearance is the highest treatment goal recommended by the current guidelines related to the prevention and treatment of Chronic Hepatitis B (CHB).1-3 This is also known as a functional cure of hepatitis B and is closely associated with improvement in long-term prognosis.4 However, a certain recurrence rate remains even after HBsAg seroclearance. Based on a prospective study of 238 patients treated with Pegylated-Interferon-α (Peg-IFNα) who achieved HBsAg seroclearance,5 the cumulative HBsAg relapse rate from the median follow-up to 160 weeks was 9.66%. A meta-analysis based on 43,924 patients showed that the HBsAg relapse rate was 6.19%.6 Recent studies have indicated that the seroconversion rate and level of anti-HBs are strongly associated with HBsAg recurrence.5,7,8 A study conducted by Li et al. showed that the recurrence rate was significantly lower in functionally cured patients who were positive for antibodies to the HBsAg (anti-HBs) than in those who were negative for anti-HBs.7 Two recent retrospective studies both suggested that the HBsAg recurrence rates were lower in functionally cured patients with anti-HBs ≥100 IU/L than in those with anti-HBs < 100 IU/L.5,9 To increase the anti-HBs seroconversion rate and antibody levels, some studies have reported that administration of hepatitis B vaccine (hereafter referred to as the vaccine) may improve treatment outcomes. In a real-world study, 20 μg of the vaccine was administered to 11 individuals who had achieved HBsAg seroclearance, and their anti-HBs seroconversion rate at the 9-month follow-up remained 81.8%, but the study did not include a control group.10 In another prospective study of 33 individuals who experienced HBsAg recurrence after functional cure with Peg-IFNα, 18 of those who received the vaccine during retreatment showed anti-HBs seroconversion, while only 26.7% of the other 15 individuals who did not receive the vaccine showed the presence of these antibodies.11
Regarding whether the use of the hepatitis B vaccine can effectively increase the anti-HBs positive conversion rate and antibody levels, there are currently few relevant studies, with inconsistent conclusions. Therefore, we conducted a retrospective study on functionally cured patients with IFNα therapy.
MethodsStudy subjectsThe study subjects were from our patients who had achieved functional cure of CHB in the earlier stage with IFNα-based therapy (mostly with Peg-IFNα, and a few with short-acting IFN) and from recently accumulated case data in our hospital.5,12 The study period was from September 2010 to January 2022. All subjects were treated at Beijing Youan Hospital, Capital Medical University, and met the criteria of age 16–65 years and at least one follow-up review within every 6-months, with complete laboratory data that focused on serological markers, HBV DNA, and transaminases. Chronic HBV-infected patients were excluded if they had contraindications related to interferon treatment, such as autoimmune diseases. They were also excluded if they had related diseases that affect antibody production, such as HIV infection, immunosuppressive conditions, tumors, etc. In this study, functional cure of hepatitis B was defined as HBsAg of < 0.05 IU/mL, negative Hepatitis B envelope Antigen (HBeAg), HBV DNA below the lower limit of detection, and normal Alanine Transaminase (ALT).
Information collection and follow-upA retrospective study approach was used. A questionnaire was distributed through an online survey platform to collect information related to hepatitis B vaccination in patients who had achieved functional cure of hepatitis B. The questions were related to whether hepatitis B vaccine had been used after HBsAg seroclearance, type and dose of vaccine, frequency of vaccination, and adverse reactions related to vaccination. The duration of IFNα therapy, duration of consolidation therapy after HBsAg seroclearance, and anti-HBs levels at different time points were obtained through our laboratory information system and from medical records.
The study was approved by the Ethics Committee of our hospital ([2018]050), and all investigated patients had signed an informed consent form before completing the questionnaire.
Laboratory tests and methodsThe serological markers of HBV were detected using Elecsys-2010 (Roche, Mannheim, Germany) with a lower limit of detection of 0.05 IU/mL for HBsAg and 2 IU/L for anti-HBs. Positive results were defined based on an HBeAg cut-off index ≥1 and anti-HBs levels ≥10 IU/L. HBV DNA was measured using the COBAS TaqMan fluorescent quantitative polymerase chain reaction system (Roche), with a lower limit of detection of 20 IU/mL. Liver function tests were performed using an OLYMPUS-AU5400 (Shinjuku, Japan) biochemical analyzer with a normal range of 9–40 U/L for ALT.
Statistical methodsSPSS v22.0 (IBM SPSS Inc., Armonk, NY, USA) was used for statistical analysis. Descriptive data are presented as mean ± standard deviation or number and percentage. The independent samples t-test was used for component comparison of normally distributed measurement data, whereas the Mann-Whitney U test was used for group comparison of skewed measurement data. Categorical variables were analyzed using the χ2 or Fisher's exact test. Logistic regression was used to analyze the influencing factors. A p < 0.05 was considered to indicate a statistically significant difference.
ResultsGeneral informationWe distributed 355 questionnaires through an online survey platform from September 2022 to October 2022 and collected 335 questionnaires, of which 320 were valid (the questionnaire and test results are complete). Of these, 194 were from male and 126 from female patients. The mean age was 38.28±9.68 years, the median duration of IFN therapy was 84 weeks, and 87.5% of the patients were on IFN consolidation therapy for more than 12 weeks after HBsAg seroclearance (Table 1).
General information of 320 patients.
HBsAg, Hepatitis B surface Antigen; HBeAg, Hepatitis B envelope Antigen; IFN, Interferon.
Hepatitis B vaccination was based on patients’ personal preferences and drug accessibility. In total, 219 patients who had undergone vaccination after HBsAg seroclearance were defined as the vaccinated group, whereas the remaining 101 who did not receive vaccination were defined as the unvaccinated group. All vaccines used were recombinant hepatitis B vaccines of 20 μg/dose (including products from GSK, London, UK; and North China Pharmaceutical Company Ltd, Shijiazhuang, China). The frequency of vaccination ranged from 1–12 doses, with a median of 9 doses, and the interval of administration ranged from 2 to 4 weeks.
Comparison of anti-HBs status between the vaccinated and unvaccinated groupsIn this study, HBsAg of < 0.05 IU/mL and anti-HBs of ≥ 10 IU/L were defined as anti-HBs seroconversion. The anti-HBs seroconversion rate of 78.1% in the vaccinated group was significantly greater than that of 41.6% in the unvaccinated group (p < 0.001). The differences in anti-HBs levels between the two groups were further compared through stratification by two levels of anti-HBs, which were ≥ 100 IU/L and ≥ 300 IU/L. The proportion of patients with anti-HBs of ≥ 100 IU/L was 71.2% and 32.7% in the vaccinated and unvaccinated groups, respectively, whereas the proportion with anti-HBs of ≥ 300 IU/L was 56.2% and 17.8%, respectively, and both were statistically different (all p-values < 0.001) (Table 2). These results suggested that vaccination may not only increase the antibody seroconversion rate but may also increase the antibody level.
Comparison of anti-HBs status between vaccinated and unvaccinated groups.
Anti-HBs, Antibodies to Hepatitis B surface Antigen.
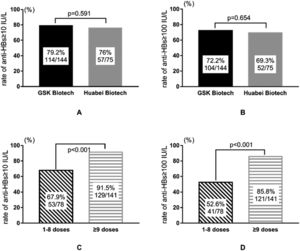
Among the 219 patients who were vaccinated, 144 and 75 individuals used the vaccines from GSK and North China Pharmaceutical Company Ltd, respectively. Differences in the seroconversion (≥ 10 IU/L) and level (≥ 100 IU/L) of anti-HBs between different types of vaccine were insignificant (all p-values > 0.05) (Fig. 1A/1B). Regarding the frequency of vaccination (regardless of the type of vaccine used), a stratified comparison was performed in terms of whether ≥ 9 doses of vaccine were used. Those who used ≥9 doses had anti-HBs seroconversion rates of up to 91.5%, and 85.8% of them had antibody levels of ≥ 100 IU/L, as compared with only 67.9% and 52.6%, respectively, for those who used 1–8 doses of vaccine (all p-values < 0.001) (Fig. 1C/1D).
Analysis of influencing factors for anti-HBs levels in the overall populationFurther analysis was performed on the possible influencing factors for anti-HBs levels in the overall population (including vaccinated and unvaccinated groups), such as sex, age, duration of IFN therapy, duration of consolidation therapy, and vaccination status. The results showed that the influencing factors were more obvious for the grouping based on reaching anti-HBs levels of ≥ 100 IU/L. Univariate analysis showed that patients who reached anti-HBs of ≥ 100 IU/L were younger and received more than 12 weeks of consolidation therapy and included a higher proportion of individuals who used vaccines. Logistic regression analysis also showed that younger age, more than 12 weeks of consolidation therapy, and vaccination were favorable factors for reaching anti-HBs of ≥ 100 IU/L. The odds ratio for vaccination was 4.427, which was the strongest influencing factor for anti-HBs reaching ≥ 100 IU/L (p < 0.001) (Table 3).
Analysis of influencing factors for anti-HBs levels in the overall population.
Anti-HBs, Antibodies to Hepatitis B surface antigen; IFN, Interferon; HBsAg, Hepatitis B surface Antigen; OR, Odd Ratio.
Among patients who received hepatitis B vaccines, nine patients experienced varying degrees of redness and mild pain at the injection site after the vaccination. These reactions were resolved within a few hours to 3 days. Another three patients developed fever (maximum temperature did not exceed 38°C) after hepatitis B vaccination, and one of them had an accompanying sporadic skin rash. The remaining patients had no significant adverse reactions after vaccine administration.
DiscussionWith the continuous optimization of antiviral treatment regimens, an increasing number of patients with CHB have achieved functional cure.12,13 However, methods to maintain such functional cure of hepatitis B and to reduce HBsAg recurrence are major clinical concerns. At present, there are no effective means to increase the seroconversion rate and level of anti-HBs. Some studies have shown that using the hepatitis B vaccine in functionally cured patients can increase the anti-HBs seroconversion rate and reduce recurrence.9-11 Recent studies have shown that functional cure appears to be more dependent on seroconversion and the anti-HBs levels in terms of maintaining the durability of HBsAg seroclearance in patients who received IFN treatment than in those who achieved a Nucleot(s)ide-Analogues (NA)-induced cure.9,14 A study conducted by Jiang suggested that, among individuals with IFN-induced functional cure, HBsAg recurrence rates were significantly lower in individuals with anti-HBs levels reaching ≥ 100 IU/L after hepatitis B vaccination than in individuals whose anti-HBs levels did not reach 100 IU/L after hepatitis B vaccination and in unvaccinated individuals (7.7% vs. 58.5% vs. 31.9%, respectively; all p-values < 0.05).9 In contrast, among individuals with NA-induced and spontaneous HBsAg seroclearance, there was no difference in HBsAg recurrence rate regardless of hepatitis B vaccination. Another study that included 70 individuals with NA-induced HBsAg seroclearance showed that anti-HBs seroconversion was not an effective protective factor against HBsAg recurrence.14 Therefore, the use of the hepatitis B vaccine to promote seroconversion and high levels of anti-HBs is important for reducing recurrence in individuals who achieved HBsAg seroclearance with IFN therapy.
To analyze the correlation of both the seroconversion rate and level of anti-HBs with the use of the vaccine, we conducted this retrospective study. We found that the anti-HBs seroconversion rate was significantly higher in the vaccinated group than in the unvaccinated group (78.1% and 41.6%, respectively, p < 0.001). In anti-HBs ≥100 IU/L and ≥300 IU/L subgroup analyses, anti-HBs levels were significantly higher in the vaccinated group than in the unvaccinated group (71.1% vs. 32.7%, and 56.2% vs. 17.8%, respectively, all p-values < 0.001). These results suggest that vaccination not only increases the antibody seroconversion rate but also increases antibody levels significantly. Our present results are similar to the results of Zeng and Wu.10,11 Our study also suggested that increasing the frequency of vaccination might increase the seroconversion rate and antibody levels significantly, but no significant difference was observed with different types of vaccines. No similar study has been reported. We further analyzed the factors that may influence antibody levels in the overall population (both vaccinated and unvaccinated groups) and found that younger age, consolidation therapy of more than 12 weeks, and vaccination were favorable factors for producing high levels of anti-HBs. Logistic regression analysis showed that vaccination was the strongest influencing factor for producing high levels of antibodies (OR = 4.427, p < 0.001). A study conducted by Li et al. found that consolidation therapy could increase anti-HBs levels and reduce HBsAg recurrence in functionally cured hepatitis B patients.7
Previous studies have suggested that patients with untreated CHB have a reduced capacity for B cell proliferation and thus are unable to produce anti-HBs,15,16 whereas the reason for the good response of patients to the hepatitis B vaccine in this study was thought to be related to the use of IFNα. The results of a previous prospective study showed that 103 patients with CHB who had HBsAg of < 1500 IU/mL showed a significant increase in the proportions of both memory B cells and plasma cells after 24 weeks of Peg-IFNα therapy.17 A study conducted by Liu et al. suggested that the proportions of follicular helper T (Tfh) cells and specific B cells among Peripheral Blood Mononuclear Cells (PBMCs) in patients who achieved HBsAg seroclearance and who produced anti-HBs increased significantly with a longer duration of Peg-IFNα therapy.18 The differences were statistically significant as compared with those who did not convert to negative for HBsAg. The authors concluded that Peg-IFNα enhances B cell function and promotes protective antibody production by increasing the number of Tfh cells. Our study obtained a higher seroconversion rate and level of anti-HBs when the hepatitis B vaccine was added to IFNα therapy after HBsAg seroclearance, presumably by the same mechanism as above. This study also showed that the seroconversion rate and level of anti-HBs varied with the frequency of vaccination, probably due to the differences in immune function between patients with CHB and the healthy population. Compared with the routine three-dose vaccination for healthy people at the ages of 0, 1, and 6 months, patients with CHB need to be vaccinated more frequently to promote the production of anti-HBs.
This study had some limitations, including its retrospective nature and limited sample size. Additionally, no study was conducted on the relevant mechanism of immune function, which will require further research.
In conclusion, the use of the hepatitis B vaccine in patients with CHB after HBsAg seroclearance not only increased the anti-HBs seroconversion rate but also increased the related antibody level significantly. The production and high level of anti-HBs can reduce HBsAg recurrence, particularly in individuals who achieved functional cure with IFNα therapy. This protective antibody is of utmost importance in this regard. In addition, no significant adverse effects were observed with the prophylactic hepatitis B vaccine in patients with CHB, and the overall safety was good, which warrants further clinical observation and application.