Leprosy reactions are an acute inflammatory phenomenon that can arise before diagnosis, during treatment, or after cure of leprosy. These reactions are considered one of the main diseases that cause physical disabilities. Immunosuppressive treatment for these immune responses makes these patients susceptible to coinfections, which can trigger new leprosy reactions. The main objective of this study was to evaluate the occurrence of infection by Bartonella sp. in blood samples from 47 patients who had untreatable episodes of type 2 leprosy reactions for more than six months, comparing them with a control group. Cultures and molecular methods (PCR) were used. Amplicons from species-specific reactions and sequencing showed a higher prevalence of Bartonella henselae infection in patients, 19/47 (40.4 %), compared to control, 9/50 (18.0 %), p = 0.0149. Five patients accepted treatment for coinfection, and all showed improvement in leprosy reactions with treatment for B. henselae infection. We conclude that these bacteria can trigger chronic reactions of type 2 leprosy and should be investigated in these patients.
Summary linePatients who have chronic type 2 leprosy reactions are more susceptible to Bartonella henselae infection than controls: 19/47 (40.4 %) compared 9/50 (18.0 %), p = 0.0149.
Leprosy is an age-old disease related to poverty because certain conditions of less favored populations considerably increase exposure to Mycobacterium leprae and Mycobacterium lepromatosis.1,2 The disease has a clinical spectrum closely related to the genetically inherited immune capacity of the affected individual. Patients with a good cellular immune response are more affected by localized forms such as tuberculoid, while those with more diffuse forms of the disease have a predominance of the humoral response, which is ineffective to combat intracellular infection in Borderline (BB), Borderline Lepromatous (BL) and Lepromatous (LL).3,4
Before, during, or even after the end of treatment, approximately 30 %‒50 % of patients develop acute inflammatory reactions to mycobacterial antigens, often located in the peripheral nerves. These inflammatory reactions are responsible for most physical disabilities related to infectious diseases.5,6 Studies show that infection by Bartonella sp. can also affect the nervous system. A mouse study suggests that B. henselae infection induces persistent mechanical hypersensitivity, and some reports show that Bartonella spp. may be a potential cause of chronic neurological and neurocognitive dysfunction, even in immunocompetent patients.7,8 Another study that investigated the atypical manifestations of Cat Scratch Diseases (CSD) showed that, in the United States, neurological manifestations (neuritis or encephalitis) were one of the most frequent (13.8 %), as well as ocular manifestations (retinitis/neuroretinitis or conjunctivitis) (48.7 %) and hepatosplenic diseases (24.6 %).9
Reactions can be divided into Type 1 (T1R) and type 2 (T2R) leprosy reactions. T2R are known as a synonym for with Erythema Nodosum Leprosum (ENL) even though these reactions may present with other clinical manifestations such as erythema multiforme, erythematous plaques or even purpuric lesions, without necessarily coursing with erythema nodosum. The T2R are usually self-limited last for approximately two weeks. The T1R should last for few weeks to few month.10 When patients have subsequent leprosy reactions, i.e., for more than six months, they are considered to have chronic reactions, and neural involvement is common. These reactions require corticosteroid treatment, often in high doses. In these cases, it is necessary to investigate factors that may act as triggers for these reactions, including coinfections, albeit subclinical.11,12
Immunity mediated by Th1 cells plays an important role in the evolution of intracellular bacterial infections and, therefore, in the evolution of leprosy and its reactions.3 There is evidence that cell-mediated immunity is also closely related to the pathogenesis and control of infection by Bartonella spp.13 These bacteria can cause asymptomatic infection and are responsible for emerging and reemerging diseases.14 At least 17 species and subspecies of Bartonella are associated with human diseases, such as Carrión disease (Peruvian bartonellosis), trench fever, bacillary angiomatosis, endocarditis, and CSD. Among other clinical manifestations, erythema nodosum has been associated with Bartonella sp.-infections.13,15,16 Often neglected, even when the diagnosis of these bacterial infections is considered, confirmation in routine microbiological laboratories is difficult. Being fastidious, they require enriched media and other special conditions to be isolated, in addition to presenting extremely slow growth.17 Similar to other Gram-negative bacteria, they present with low bacteremia, making molecular diagnosis from blood samples difficult.18
Among Bartonella spp., Bartonella henselae is the species most frequently associated with human diseases.19-21 Similar to other pathogenic species, it is transmitted by blood-sucking ectoparasites, particularly the flea (Ctenocephalides felis), with its main host being domestic cats.22 Coinfection by B. henselae in a patient with chronic leprosy reactions monitored at Clinics Hospital of UNICAMP has already been described. In this patient, there was complete improvement of the reactions with the treatment for B. henselae.23 Other cases of the association of leprosy with B. henselae have also been cited in an article on the detection of bacterial DNA in paraffin material.24
The main objective of this study was to evaluate and compare the occurrence of infection by Bartonella sp. in blood samples from patients from two reference centers for the treatment of leprosy in Southeastern Brazil with a control group of individuals without clinical complaints.
Material and methodsBecause leprosy is a moderately endemic disease in the Southeastern Region of Brazil,25 patients from two reference centers for leprosy treatment participated in the study: the Dermatology Outpatient Clinic of the Clinics Hospital of UNICAMP, Campinas-SP, Brazil and the National Reference Center for Leprosy and Sanitary Dermatology (CREDESH), Uberlandia-MG, Brazil. Many leprosy patients with chronic reactions are referred to these centers. The selected patients presented difficult-to-manage T2R with reactions for more than six months; the control group, students, and employees at UNICAMP, did not mention clinical symptoms at the time of sample collection. All study participants were older than 18 years and not pregnant.
The project was approved by the Institutional Research Boards of the State University of Campinas (UNICAMP), Campinas, SP (CAAE: 44,670,015.4.0000.5404) and the Federal University of Uberlandia (UFU), Uberlândia, MG (CAAE: 44,670,015.4.3001.5152), with both institutions located in the Southeastern region of Brazil (22°49′36.0″S 47°03′50.9″W and 18°53′36.2″S 48°17′45.5″W, respectively) (Fig. 1).
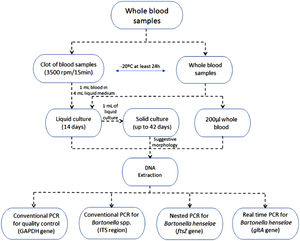
A volume of approximately 8 mL of blood without anticoagulant was collected in an aseptic manner in a tube from each participant and another 8 mL in a tube containing EDTA (ethylenediaminetetraacetic acid). The blood clot obtained from the dry tube after centrifugation at 3500 rpm for 15 min, and the tube containing blood with EDTA were frozen at −20 °C for at least 24 h.
After freezing, 1 mL of whole blood was inoculated into 4 mL of liquid medium specific for the growth of Bartonella spp.,26 and the same was done with 1 mL of the clot. Both cultures were incubated in a shaker at 35 °C with 5 % CO2 for 14 days.
After 14 days, 1 mL of the liquid culture was seeded in a solid medium as previously described.18 The culture flasks were kept at 35 °C with 5 % CO2 in a water-saturated atmosphere for up to 42 days. Evaluations verifying growth were performed weekly. When there was bacterial growth, a colony was collected and stained using the Gram technique. If Gram-negative bacteria suggestive of Bartonella sp. were observed, samples were collected for subsequent molecular analysis.
DNA extraction was performed with the commercial QIAamp DNA Mini Kit (Qiagen) according to the manufacturer's recommendations using whole blood, liquid culture (whole blood and clot), and solid culture isolates.
All extracted samples, except for the isolates, were tested with PCR for amplification of the gene fragment GAPDH (glyceraldehyde-3-phosphate dehydrogenase) related to the production of an enzyme expressed by mammalian cells for the purpose of verifying the quality of the extracted DNA and to rule out the existence of amplification inhibitors.27
The DNA of Bartonella sp. was amplified by PCR from whole blood, blood liquid culture, clot liquid culture and subculture isolates in solid medium from liquid cultures. Three types of reactions were used: conventional PCR, nested PCR and qualitative real-time PCR.
In conventional PCR, primers for the genus Bartonella were used, directed to the Intergenic Spacer region (ITS) 16S-23S of the rRNA.28 In nested PCR, primers were used for the target gene which encodes the ftsZ protein involved in bacterial cell division, which is species-specific for B. henselae.29 In real-time PCR, primers were used for the target gene which encodes an enzyme which encodes the citrate synthase (gltA) in the SYBR Green system, also specific for B. henselae, and these results were used only qualitatively, considering the results as positive or negative.30
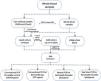
All PCR products were analyzed by electrophoresis in a 2 % agarose gel stained with GelRed® and visualized under ultraviolet light. The flowchart of the procedures is shown in Fig. 2. The obtained amplicons that showed quality for sequencing were sent for comparison with previously deposited genetic material.
ResultsThe sensitivity of the reactions was 50 equivalent genomic copies per tube for conventional, genus-specific PCR, and 10 for species specific, nested PCR and real-time PCR. A total of 47 patients participated in the study, of which 20 were undergoing treatment at UNICAMP and 27 at CREDESH. The control group consisted of 50 volunteers, students or employees of UNICAMP. DNA of B. henselae was detected in at least one sample of 19 of the 47 patients (40.4 %), while in the control group, it was detected in 9 of the 50 volunteers (18,0 %). There was a statistically significant difference (p = 0.0149) using the chi-square test (Table 1).
Results of DNA detection of Bartonella sp. per analyzed group.
| Groups | Patients | Controls | p-value | |
|---|---|---|---|---|
| UNICAMP | CREDESH | |||
| Total | 20 | 27 | 50 | – |
| DNA detection | 8 | 11 | 9 | – |
| Detection rate (%) | 40.0 % | 40.7 % | 18.0 % | 0.0149 |
UNICAMP, Patients followed up at the Clinical Hospital of the University of Campinas, Campinas-SP; CREDESH, National Reference Center for Leprosy and Sanitary Dermatology, Uberlandia-MG.
DNA from B. henselae was detected in 40.0 % of UNICAMP patients and in 40.7 % of CREDESH patients. There was no significant difference in the detection of bacterial DNA between the two groups (p = 0.9591).
Considering that chronic leprosy reactions are a rare event6 and using Fisher's exact test to establish the sample calculation of the study and setting the significance level at 5 % (alpha or type I error) with a sample power of 80 % (beta or type II error of 20 %), based on the data obtained from the UNICAMP patients, it can be established that the minimum sample for the results to have statistical significance would be 42 patients.
Of the 28 individuals, patients and healthy volunteers, who had B. henselae-DNA detected in the molecular tests, five had B. henselae-DNA detected in species-specific reactions but could not have their amplicons sequenced, and 23 had at least one of their samples with sequenced amplicons. All sequenced samples showed 99 % to 100 % similarity for B. henselae. The access code corresponding to GenBank® was KT945243.1 in the gltA gene, HG965802, in the ftsZ gene and CP020742.1 in the ITS region. The bacteria were isolated in four patients and in two controls.
DiscussionOur data show the association of the B. henselae-bloodstream infection and chronic T2R. This infection is not consistently self-limiting, and a subset of asymptomatic individuals can be bacteremic, potentially for long, but poorly defined periods. Bartonelloses can also presented by atypical manifestations other than CSD, bacillary angiomatosis/pellioses, Peruvian bartonellosis, or endocarditis and are neglected diseases once neglected diseases can be potentially controlled, prevented and even eradicated using feasible and effective measures. Bartonelloses are not included in the World Health Organization list of Neglected Diseases, but, similar to leprosy, they are supposedly to be more prevalent in vulnerable populations.31Bartonella sp. can cause subclinical infection and thus, theoretically, trigger leprosy reactions, which are associated with physical disabilities resulting from these reactions.14
The prevalence of Bartonella sp. infection in asymptomatic humans has been evaluated by molecular tests in some studies and ranges from 1.8 % to 23 %,14,32-36 but different methodologies have been used, and these results cannot be compared to each other. Portillo et al. analyzed samples from 97 health professionals using several molecular tests from whole blood, liquid and solid cultures in addition to sequencing. With this combination of diagnostic tests, they obtained the DNA of Bartonella spp. was amplified by 21.6 %.37 This percentage of molecular detection in asymptomatic individuals is very similar to Drummond et al. whose study analyzed 500 blood donors and the agent was detected or isolated in 23 % of the analyzed blood donors.36 In the present study, B. henselae-DNA was detected in nine of the 50 (18.0 %) volunteers, who did not report symptoms, in at least one of the molecular reactions.
There are few studies that have evaluated the detection of Bartonella sp. in groups of patients with other diseases. In patients with rheumatic symptoms, Bartonella sp. infection was observed by PCR analysis of blood, serum and BAPGM liquid culture samples and isolates in 41.1 % of 296 participants.38 The risk of developing Bartonella sp. infection was 22 times higher in patients with endocarditis, 45 times higher in patients with arrhythmias and 40 times higher in patients with chagasic myocarditis than in a group of asymptomatic volunteers.32 There was no statistically significant difference between a small group of patients with psoriasis and healthy individuals, although B. henselae-DNA was detected in 6/30 and 3/30 individuals, respectively.34 A recent study using digital PCR with patients with schizophrenia showed a statistically higher prevalence (p = 0.0024) compared to healthy volunteers, with detection in 11 of the 17 patients and in one of the 13 individuals in the control group.33 Patients with primary livedoid vasculopathy had twice as much B. henselae infection, 4/16 (25 %) compared to the control group, 4/32 (12.5 %), but the difference was also not statistically significant (p = 0, 24).39 These data demonstrate that not all individuals colonized by B. henselae present clinical manifestations, typical or not, and that the chronic inflammation caused by the presence of the bacteria can be one of the pathogenic mechanisms in the triggering of diseases such as the chronic leprosy reaction or other immune mediated diseases.
Of the two services researched in the Southeastern region of Brazil, there was no significant difference between the group of patients with chronic leprosy reactions. In this region, the rate of patients with permanent sequelae disabilities is greater and higher than in regions of the country where the rates of leprosy detection are higher.23,25 Infection with B. henselae may be indirectly associated, among other factors, with physical disabilities in patients with leprosy by triggering leprosy reactions.
B. henselae was isolated from patients and controls. Although B. henselae is the species most associated with diseases in humans,21 in the present study, all samples were tested with three reactions, but just the conventional PCR was genus-specific, which has lower sensitivity than the two species-specific reactions used (nested and real-time). This factor could skew the results for the detection of B. henselae-DNA exclusively in all individuals with the Bartonella sp.-DNA detection.
The previously described patient with M. leprae and B. henselae coinfection had two blood samples collected during retreatment with MDT for leprosy with detection of Bartonella sp.-DNA in both samples.23 In this case of coinfection, the patient had been treated for leprosy with 24 doses of the MDT regimen (in which dapsone had been replaced by ofloxacin by anemia), maintained chronic T2R for 33 months after the end of this first treatment and had started retreatment with the same regimen for six months when B. henselae infection was effectively treated. The patient completed 12 doses for his multibacillary leprosy and had no recurrence of leprosy, even after 60 months of follow-up after B. henselae treatment.23
The need for retreatment occurs in 11.9 % of cases of CREDESH, 25.45 % of UNICAMP patients and 15.1 % of the cases in Campinas in 2018.40,41 The role of B. henselae coinfection in the need for retreatment for leprosy needs to be better evaluated in due course.
There are several reports supporting that therapeutic elimination of Bartonella spp. it is difficult and questionable.42,43 In Carrión disease caused by Bartonella bacilliformis, therapeutic failures and persistent bacteremia have been reported, and successful treatment of Oroya fever with antibiotic does not eliminate the patient's risk for development of the verruga Peruana.44 There are reports of cases of recurrence of trench fever, caused by Bartonella quintana, years after treatment of the infection.45 and doxycycline and were treated with azithromycin orally for six weeks. The eradication of Bartonella sp. from the human body with antibiotic treatment is questionable.
There are no studies on the ideal treatment for diseases caused by Bartonella sp. other than CSD, for which the use of azithromycin orally for five days is indicated.46 Spach suggests treatment for endocarditis by Bartonella sp. with gentamicin and doxycycline for two and six weeks, respectively. The alternative use of azithromycin for up to six months is cited by the same author.47 Future studies are needed to define the best treatment for B. henselae infection in coinfection with M. leprae.
ConclusionsChronic T2R patients may be more related to B. henselae-DNA detection than asymptomatic individuals.
FundingDoctoral scholarship by The Brazilian National Council for Scientific and Technological Development (CNPq)170501/2018-3 (Santos, LS); Postdoctoral Scholarship by The São Paulo Research Foundation (FAPESP)2018/12565-6 (Drummond, MR); Productivity Grant by The Brazilian National Council for Scientific and Technological Development (CNPq)306970/2018-0 (Velho, PENF). Research Grant by the São Paulo Foundation against Leprosy193/2018. Support Fund for Dermatology of São Paulo (FUNADERSP). Fund for Support to Teaching, Research and Outreach Activities (FAEPEX) 3184/18.
We thank the National Council for Scientific and Technological Development (CNPq), the São Paulo Research Foundation (FAPESP), the São Paulo Foundation against Leprosy and Fund for Support to Teaching, Support Fund for Dermatology of São Paulo (FUNADERSP), and Research and Outreach Activities (FAEPEX) for their financial support.