The transmission of diseases by blood products continues to be a worldwide health problem, especially in Africa. Seroprevalence rates of the Hepatitis B virus (HBV), Hepatitis C virus (HCV), Human Immunodeficiency Virus (HIV), Syphilis, and Coinfection in Angola are poorly documented. This study aims to identify the seroprevalence of markers with positive results for Hepatitis B, C, HIV, Syphilis, and Coinfection in blood donors.
Material and methodsA retrospective study was conducted using a database of positive serological markers for these infections and coinfection in 2734 blood donors traced from 2011 to 2016 in Luanda, Angola. The Chi-Square test (χ2) or Fisher's exact test was used to evaluate serological positivity and donors’ characteristics. A p-value < 0.05 was considered statistically significant.
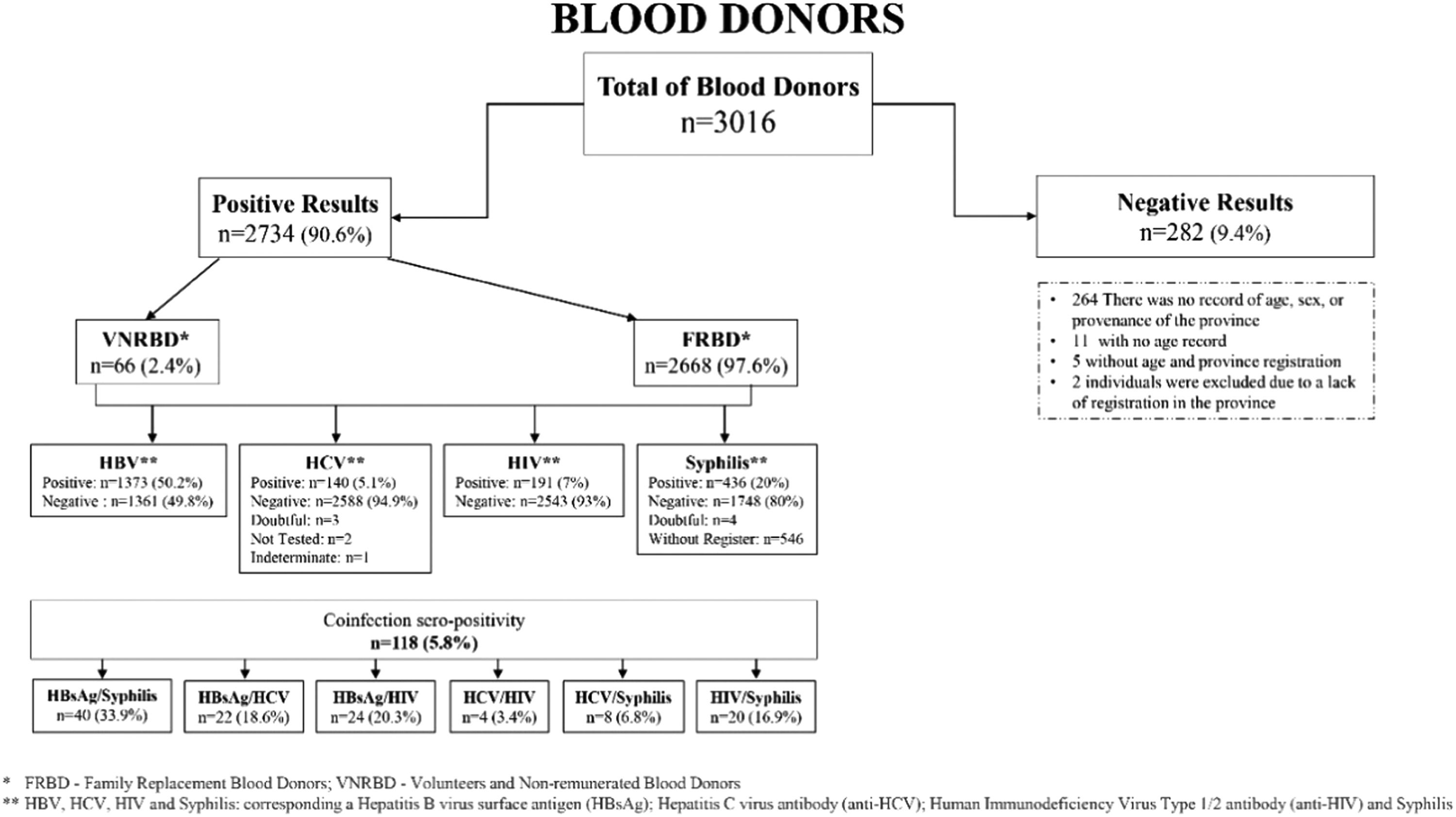
Results2734 blood donors aged 18 to 64 (median age 32 ± 9) were screened from 2011 to 2016. 73.9 % of the donors were positive for one Transfusion-Transmitted Infection (TTI), and 5.9 % showed evidence of multiple infections. The overall seroprevalence rate was 50.2 % (1373) for HBV, 20 % (436) for Syphilis, 7 % (191) for HIV, 5.1 % (140) for HCV, and 5.8 % for coinfected donors. 2467 (90 %) were men, and 267 (10 %) were women. We identified 118 (5.8 %) coinfected donors. Of those, 40 (33.9 %) simultaneously presented Hepatitis B virus surface antigen (HBsAg)/Syphilis, 24 (20.3 %) HBsAg/HIV, 22 (18.6 %) HBsAg/HCV, 20 (16.9 %) HIV/Syphilis, 8 (6.8 %) HCV/Syphilis, and 4 (3.4 %) HIV/HCV.
ConclusionA high transfusion-transmissible infection prevalence was found compared to some countries in Sub-Saharan Africa. Therefore, intensifying the screening for these transfusion-transmitted infections in blood donors is critical to ensure blood safety.
In Southern Africa, safety in blood transfusion and its derivatives remains compromised by multiple and diverse factors and is affected by a high prevalence of transfusion-transmitted infections. Infections such as Hepatitis B, Hepatitis C, and HIV are of great concern because of their prolonged viremia and latent status.1 It is believed that the risk is high in countries of Sub-Saharan Africa, which is why the World Health Organization (WHO) has implemented standards for safe blood transfusion.2,3 Previous studies conducted in Angola to determine the prevalence of transfusion-transmitted infections, such as Hepatitis B virus (HBV), Hepatitis C virus (HCV), Human Immunodeficiency Virus (HIV), and Syphilis among blood donors, demonstrated a high prevalence of HBV, HCV, and Syphilis with 9.3 %, 8.1 %, 8.8 % and 4.6 %, respectively. For coinfection, the prevalence was 2.3 % for HIV/HBV, 0.9 % for HIV/HCV, 0.9 % for HBV/HCV, and a lower percentage for Syphilis coinfection with remaining viruses.4
Our study focused on determining the prevalence of transfusion-transmitted infections (HBV, HCV, HIV, and Syphilis) among blood donors in Angola while also investigating any correlation between the donors’ sociodemographic characteristics, the transfusion-transmitted infections addressed in this study and the presence of coinfection.
In the present study, seroprevalence for Hepatitis B virus surface antigen (HBsAg), Hepatitis C Virus antibody (anti-HCV), Human Immunodeficiency Virus Type 1/2 antibody (anti-HIV), Syphilis, and coinfection in volunteer blood donors was performed at the Clínica Girassol, in Luanda, from January 2011 to June 2016.
Materials and methodsThis retrospective study analysed the records of blood donations made by new and regular donors in Luanda from 14 January 2011 to 30 June 2016. All blood donors were healthy and non-remunerated volunteers. The study was conducted at the Girassol Clinic in Luanda (Fig. 1), which is responsible for their blood donor recruitment, collection and testing. The clinic's Immuno-hemotherapy and Blood Bank service receives donors from various regions of the country.
Map of Africa showing the location of Angola (on the right), the city of Luanda (arrow) and Clínica Girassol (on the left).5
Clínica Girassol is a private-public health institution located in the province of Luanda, the capital of Angola, a country with 18 provinces in the southern region of Africa. Angola is bordered to the north and northeast by the Democratic Republic of Congo and Congo Brazzaville, to the east by Zambia, and the south by Namibia and Botswana. To the west, it is bathed by the Atlantic Ocean (Fig. 1). According to the United Department of Economic and Social Affairs of the United Nations, the Angolan population in 2022 corresponded to 35 588 987 inhabitants5,6 and Luanda, the capital, with a current population of 7.3 million.7 Many individuals from other provinces resort to the clinic for better medical care. The centre has many departments, sections, and sub-sections. It comprises a donor, laboratory, Immunohematology, and component preparation sections. The National Blood Transfusion Service reviews the programme regularly. The collection of markers is safe, and the laboratory analyses them automatedly with high specificity and sensitivity. In the blood donation process, various methods are used to raise awareness among people, individually or in groups and involving families, about the importance and necessity of donating blood to enable treatments and therapeutic procedures for those in need. An increasing number of donors with screening indications are turning to this institution to donate blood to contribute to improving blood stocks nationwide.
All blood donors were healthy and non-remunerated volunteer blood donors. All donors conducted by the transfusion-transmitted infections section to test for Hepatitis B, Hepatitis C, HIV, and Syphilis were performed. The information from the database included coded donor ID (identification), age, sex, year of collection, type of donation, and Nationality. The outcome variables were HBV, HCV, HIV, Syphilis seropositivity and overall transfusion-transmitted infections as well as co-infections.
Pearson Chi-Square test for statistical difference in the distribution with each group was used. MI (Multiple Infection) corresponds to dual or co and triple infections. Doubtful, without register and missing data were excluded. Clínica Girassol provides health services to the locals and the population coming from different zones of the region. Doubtful results are serological results which, according to the reference value, are between 0.8‒0.9 and which, after repeating the test, remain at the same values, so the blood with these results is discarded.
Statistical analysisThe database was transferred from Excel, and the analysis was performed using the statistical analysis program SPSS®v.24.0 (Statistical Package for the Social Sciences). Categorical variables are described using absolute and relative frequencies, and continuous variables are described by the mean and standard deviation or by medians and percentiles as a function of the symmetry of their distribution. No sample size was calculated. It was a convenience sample between two available dates. The prevalence of transfusion-transmitted infections was expressed in percentages per year. The Chi-Square test (χ2) was used to evaluate the relationship between categorical variants and was applied to examine year-by-year variation. A significance level of α = 5 % was considered in all hypothesis tests.
This study follows the STROBE Statement. Permission to conduct this study and ethical clearance was obtained from the Research Ethics Committee of the National Institute of Public Health of the Ministry of Health of Angola (Reference 25/2017 and 04/2018). Written permission was obtained from the National Institute of Public Health of the Ministry of Health of Angola. The confidentiality of the information extracted from the participants’ records was maintained by not recording any personal identifiers. The data extracted were secured and made available only to the study's principal investigator. All donors signed an informed consent form (Reference FOCHBS0065) before blood collection, and blood donors were analysed anonymously. All methods were carried out in accordance with the Declaration of Helsinki.
ResultsStudy populationThe study included 2734 blood donors who were screened and donated blood from 14 January 2011 to 30 June 2016. All the participants were Volunteers and Non-Remunerated Blood Donors (VNRBD). Some were Family Replacement Blood Donors (FRBD), i.e., individuals who donated blood because they had a family member or friend hospitalised in the institution who needed blood (2668). Other donors were volunteers, i.e., individuals who were aware of the importance of blood donation to save lives and donate selflessly (66).
The blood donors were selected based on pre-set criteria based on age (18 ‒ ≤ 65 years) and weight (≥ 50 kg). For clinical screening, a standard clinical history form was provided to be filled in and used for donor selection according to the institutional protocol. According to institutional eligibility criteria, for individuals to donate blood, they must not have a history of high-risk sexual behaviour or practice, must have never received a blood transfusion, must not have jaundice, fever, or hypertension and must have never had hepatitis or surgery, among other criteria.
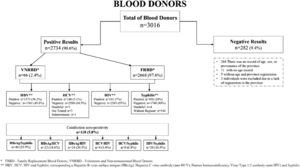
Two thousand seven hundred thirty-four (2734) donors tested positive for the Hepatitis B virus surface antigen (HBsAg), Hepatitis B virus core antibody (Anti-HBc), Hepatitis C virus antibody (anti-HCV), Human Immunodeficiency Virus Type 1/2 antibody (anti-HIV) and Syphilis infection markers. Two hundred eighty-two (282) donors did not present seropositivity for the 4 most frequent markers previously mentioned. Venous blood and plasma were collected for screening for HBsAg, anti-HCV, anti-HIV and Syphilis (Fig. 2).
Database and blood collectionThis is an institutional database with national impact, where all the data related to the internal process of each donor is recorded and stored through a code assigned to each donor, allowing this information to be analysed later. For our study, a database was created in Excel, and a structure was defined for the data where only the variables of interest to our study were entered, which refer to transfusion-transmitted infections, namely HBV, HCV, HIV and Syphilis. Concerning screening for Human T-Lymphotropic Virus (HTLV) infection, it wasn't our aim to talk about it, but it could be a topic for discussion in a future publication, as could malaria, an endemic condition in our country.
Venous blood and plasma were collected from volunteer donors (everyone, voluntary non-remunerated blood donors and family blood donors) and their relatives in tubes, which were used for screening for HBsAg, anti-HCV, anti-HIV and Syphilis, according to blood collection regulations and standards.
SerologyThe Blood transfusion service of the Immuno-Hemotherapy service of Clínica Girassol meets the guidelines for mandatory blood donor screening. Enzyme-linked Immunosorbent Assay (ELISA) was used for the screening of blood of the HBV, HCV, HIV antibodies and Syphilis using the automated Treponema pallidum Hemagglutination Assay (TPHA) and then repeated using VDRL (Venereal Disease Research Laboratory). Three confirmatory tests were performed. Two tests were used in parallel. Then, a third confirmatory test was used for discordant results.
Screening of HBsAg, antibody HCV, antibody and HIV I/II antigenScreening of Hepatitis B virus surface antigen (HBsAg) was performed using the Enzyme-linked immunosorbent assay (ELISA) kits ARCHITECT plus i1000Sr Abbott (ARCHITECT AgHBs Qualitative Test Kit). Test procedures and interpretation of results are done according to the instructions in the manufacturer's manual. Samples with questionable results, i.e. grey zone, are repeated, using the same Kit and other methodology, confirming the result in the Cobas and 601 Roche. Repeatedly positive samples were considered Hepatitis B virus surface antigen and Hepatitis B virus core antibody (AcHBc) positive. Regarding the Hepatitis C virus antibody (anti-HCV) and Human Immunodeficiency virus antibody (anti-HIV), the grey zone was repeated using the same Kit and other methodology, confirmed by the ADVIACentaur XP Immunoassay System.
Anti-Syphilis antibody screeningAntibody detection (IgG and IgM) for Treponema Pallidum (TP) was performed using the Enzyme-linked immunosorbent assay (ELISA) kits ARCHITECT plus i1000Sr Abbott. Test procedures and interpretation of results were done according to the instructions in the manufacturer's manual. The dubious grey zone results were repeated using the manual method TPHA/VDRL, the latter being considered the most specific procedure for confirming the test as a final result.
All tests used for screening have sensitivity and specificity above 99 %, according to the manufacturer, with regular quality control. The Laboratory of the Immuno-hemotherapy Service is certified by the Certified IQNet Management System.
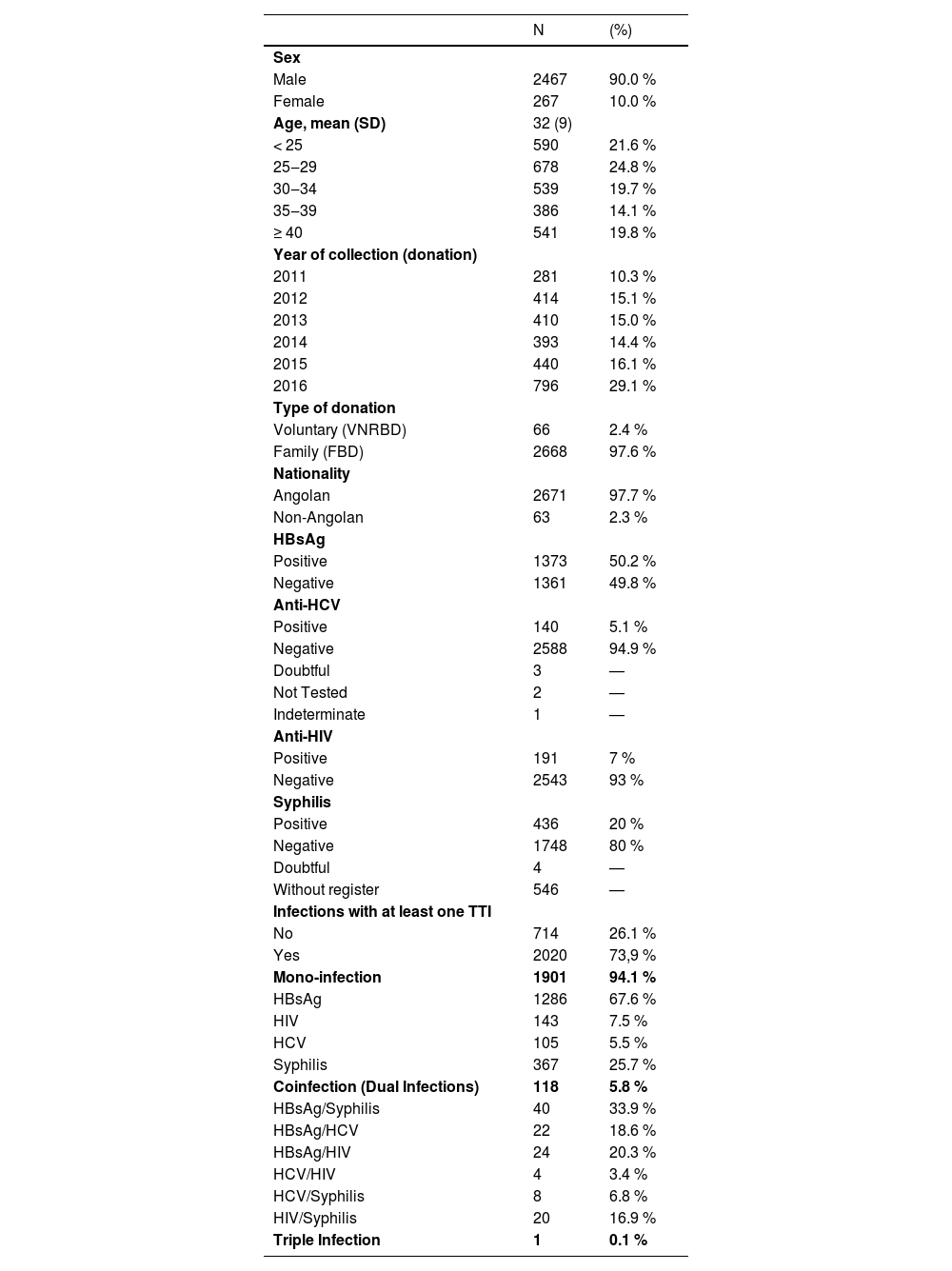
Sociodemographic characteristics and time of donorsTwo thousand seven hundred thirty-four (2734) donor samples were retrieved and analysed. The year with the most donations was 2016. The characteristics of the study population are shown in Table 1 according to the total distribution of the donors and the seroprevalence of the four transfusion-transmitted infections. Most donors were male, aged 18‒64 years old, with a mean age of 32±9 years.
Sociodemographic Characteristics of blood donors of Clínica Girassol from June 2011 to June 2016. Prevalence of TTIs (n = 2734).
Dotted lines (—) correspond to a remaining donation. The results were negative for the four TTIs tested, being doubtful, untested and undetermined in 5 donors in HCV antibody determination. Syphilis was identified as a failure in the registries. In 546 donors, there was no record of Syphilis serologies, and in 4 donors, the results were doubtful (serological results which, according to the reference value, are between 0.8‒0.9 and which, after repeating the test, remain at the same values, so the blood with these results is discarded). Voluntary Non-Remunerated Blood Donors, Family Blood Donors. Hepatitis B Virus surface Antigen (HBsAg), Hepatitis C Virus antibody (anti-HCV), Human Immunodeficiency Virus Type 1/2 antibody (anti-HIV).
Among the donors, 73.9 % (95 % CI 72.2‒75.5) tested positive for at least one transfusion-transmitted infection. The data shows that the highest proportion of donors was most notable in the 25‒29 age group, which makes up 24.8 %. Most individuals gave blood as Family Blood Donors (FBD), i.e., 97.6 %, while Voluntary Non-Remunerated Blood Donors (VNRBD) accounted for the remaining proportion, 2.4 %. Despite being low, foreigners also contributed to a small blood donation, representing about 2.3 %.
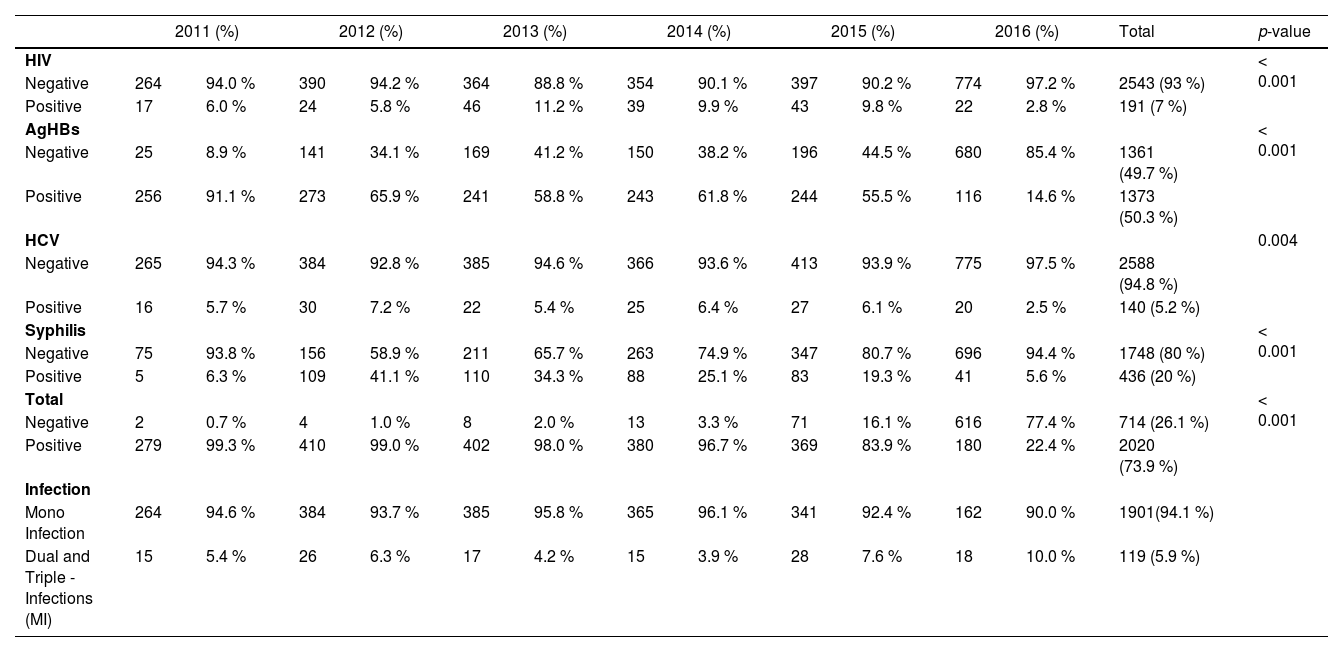
Table 2 summarises the positivity rate of transfusion-transmitted infections among donors by year. Hepatitis B was the most prevalent type throughout the 5 years. The overall prevalence of transfusion-transmitted infections fluctuated during the study period. The Chi-Square test showed a significant decrease (p < 0.001) over the 5 years. Tables 3 and 4 show the seropositivity of transfusion-transmitted infections within specific sociodemographic categories, including sex and age.
Transfusion Transmitted Infections (TTI) positivity rate among the donors by year (June 2011 ‒ June 2016).
Hepatitis B virus surface Antigen (HBsAg), Hepatitis C virus antibody, Human Immunodeficiency Virus Type 1/2 antibody (anti-HIV). p-value, Pearson Chi-Square test for statistical difference in the distribution with each group. MI (Multiple Infection): corresponds to dual or co and triple infections.
Transfusion-Transmitted Infections (TTI) positivity rate among the donors by sex.
Hepatitis B virus surface antigen (HBsAg), Hepatitis C virus antibody, Human Immunodeficiency Virus Type 1/2 antibody. p-value, Pearson Chi-Square test for statistical difference in the distribution with sex. MI, Multiple Infections, corresponds to dual or co and triple infections.
Transfusion-Transmitted Infections (TTI) positivity rate among the donors by age group.
Hepatitis B virus surface antigen (HBsAg), Hepatitis C virus antibody, Human Immunodeficiency Virus Type 1/2 antibody. p-value, Pearson Chi-Square test for statistical difference in the distribution by age group. MI (Multiple Infection): corresponds to dual or co and triple infections.
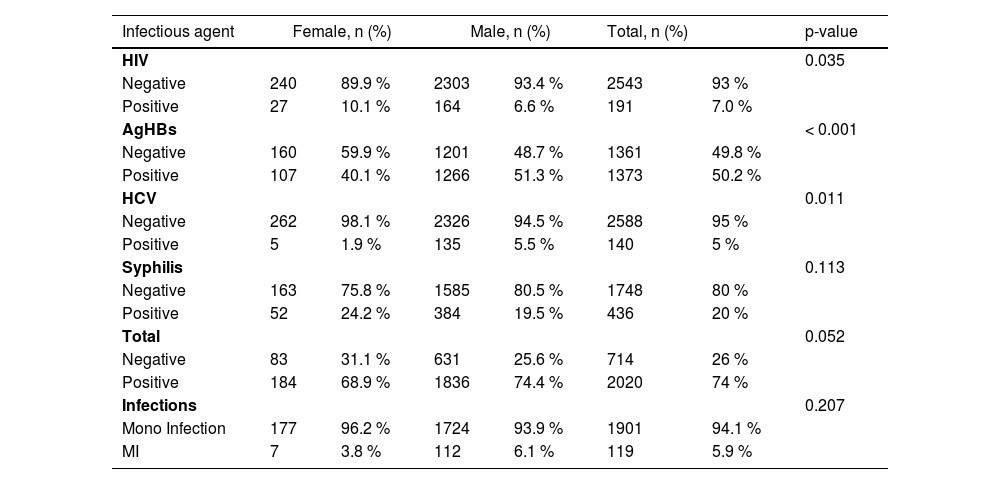
Male donors had a higher frequency of transfusion-transmitted infections than female donors, but this was not statistically significant. Female donors had a significantly higher frequency of HIV (p < 0.035) and Syphilis (p < 0.11). In contrast, male donors had comparatively higher frequencies of HBV (p < 0.001) and HCV (p < 0.011). Multiple Infections were observed at higher frequency in male donors but without statistically significant.
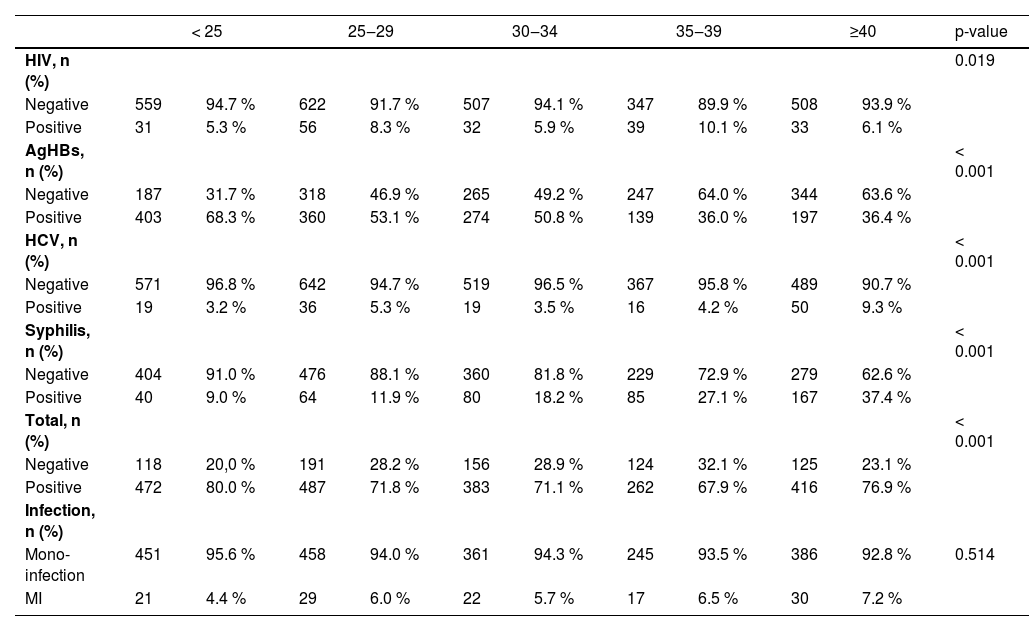
We also observed an association with a statistically significant transfusion-transmitted infections seropositivity (p < 0.001) between age categories (Table 4). In contrast, Syphilis was higher in donors aged 35‒39 and ≥ 40 years. Markers for Hepatitis C were more prevalent in donors 25‒29 and ≥ 40 years. Family Blood Donors had a higher frequency of transfusion-transmitted infections than Voluntary Non-Remunerated Blood Donors (97.6% vs. 2.4 %). The prevalence of transfusion-transmitted infections markers was significantly higher in Family Blood Donors compared to Voluntary Non-Remunerated Blood Donors: (50.4% vs. 40.9 %, p < 0.126 for Hepatitis B); for Hepatitis C (5.2% vs. 3.0, p < 0.774); for HIV (7.1% vs. 3.0 % p < 0.323) and Syphilis 20% vs. 19.2 %, p < 0.894, but not a statistically significant. The frequency of coinfection was significantly higher in Family Blood Donors (74.2% vs. 62.1 %) and statistically significant p < 0.028.
The trend of hepatitis B, hepatitis C, HIV and SyphilisThe prevalence of Hepatitis B, hepatitis B virus core antibody (anti-HBc) and HIV was statistically significantly different by year (p < 0.001), and the years with the highest record were 2012 for HBV, 2013 for HIV and 2016 for anti-HBc. However, the prevalence of HCV was relatively similar in most of the years and declined in 2016. The prevalence of Syphilis, which increased in most years, was observing its highest record in 2012, and the difference in the prevalence of Syphilis was statistically significant by year (p < 0.001). Multiple Infections were statistically significant by year, but they started to increase by 2015 (Table 2). The overall difference in the prevalence of transfusion-transmitted infections was statistically significant by year (p < 0.001). The frequency of anti-T. pallidum antibodies were higher in donors above 35‒39 and ≥ 40 years and lowest in donors aged < 25 and 34, as shown in Table 4.
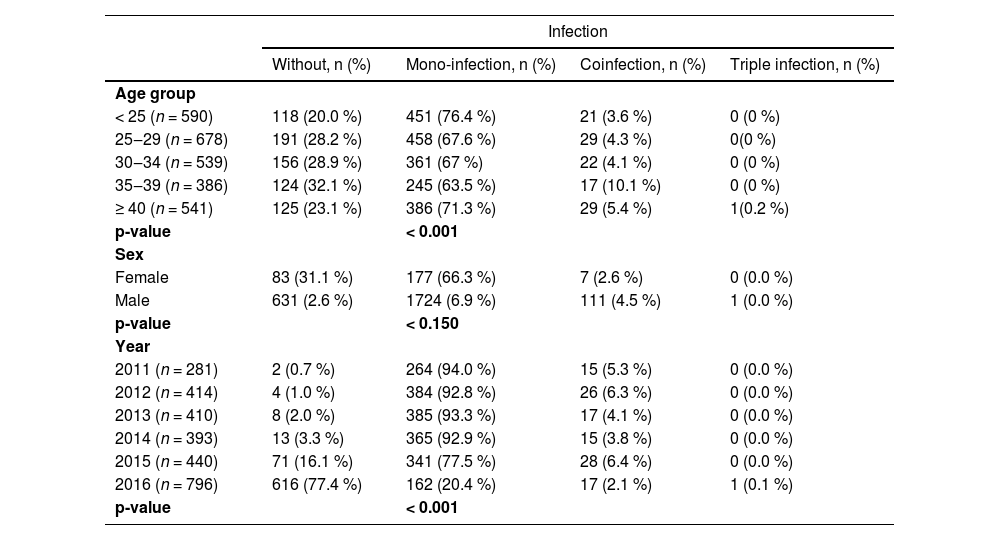
CoinfectionFive point nine (5.9 %) of blood donors had multiple infections, 5.8 % had coinfections, and 0.1 % had triple infections. Of the coinfected donors, the most dominant were positivity Hepatitis B virus surface antigen (HBsAg+)/Syphilis and HBsAg+/HIV+. A single donor presented triple infection, with positivity for HBsAg+, anti-HCV+ and anti-HIV+. The most prevalent coinfections are Hepatitis B/Hepatitis C and HIV/Syphilis.
A statistically significant association was also observed between age categories in coinfected blood donors and transfusion-transmitted infections seropositivity (p < 0.001). The data shows that among the population of blood donors according to age group, it is evident that the coinfection was more prevalent in the age group of 35‒39 years and donors above 40 years. Males in mono-infections constituted most coinfections of the replacement donors who had at least one transfusion-transmitted infection, and females made up slightly less than males among volunteer donations, but this was not statistically significant (p < 0.15). The year-to-year fluctuations in the overall prevalence of coinfections of transfusion-transmitted infections were all statistically significant (p < 0.001) Table 5.
Prevalence of coinfection among the population of blood donors according to age group.
There was a significant difference in coinfection with Hepatitis B, Hepatitis C, HIV and Syphilis concerning age groups and a year of a collection with a p-value < 0.001, but no statistical significance was observed in sex (p < 0.150). 2016 was the year with the highest blood donation, and they did not present any infection, and 2011 was the year that the donors had at least one transfusion-transmitted infection.
DiscussionThe overall cumulative frequency of transfusion-transmitted infections markers in blood donors was 73.9 %, of which HBV, HCV, HIV and Syphilis account for 50.2 %, 5.1 %, 7 %, and 20 %, respectively. The results demonstrate a higher prevalence of transfusion-transmitted infections than previous studies in Angola, which reported an overall transfusion-transmitted infections prevalence of 25.5 %4 and 18 %8 in the general population and 66.5 % in women undergoing antenatal care.9 This study showed an increase, with 50.2 % for HBV and 5.1 % HCV, and when compared to a study carried out in Namibia and Ethiopia, showed a low prevalence rate of 1.3 %10 and 11.5 %, respectively.11 The Hepatitis B, Hepatitis C, HIV, and Syphilis markers were more prevalent in men with 90 %, and this is observed in the other studies in different sub-Saharan African Countries, Nigeria,12 Malawi,13 Sierra Leone,14 Democratic Republic of Congo,15 Cameroon,16 Ethiopia.11 A lower percentage of positive transfusion-transmitted infection markers was found in females; young adults are the most prevalent age group.
A high prevalence was observed in Family Blood Donors, with 97.6 % compared to Voluntary Non-Remunerated Blood Donors. Another study conducted on the Egyptian population observed a rate of 87.7 % for Family Blood Donors,17 Eastern Ethiopia at 98 %,11 India at 85.2 %18 and Thailand17 at 71 %.
Hepatitis B was the most prevalent type of transfusion-transmitted infection throughout the 5 years, with 50.2 %. This data shows a low prevalence when compared to other studies conducted in other African countries, such as Tanzania at 86.9 %19 and a high prevalence when compared with Nigeria at 26.6 %,20 South-West Nigeria at 18.6 %,12 Mali 13.9 %.21 Other countries of Africa with a similarly high seroprevalence of HBV in BD are, for example, Benin, with 46.83 %22 and over 20 % Tunisia.23
On the other hand, 731 (62,9 %) donors were positive for Hepatitis B core antibody (anti-HBc), which, nonetheless, is lower than a study conducted in Kinshasa, with 70.9 % of anti-HBc prevalence.24 The HBV-OBI (Occult Hepatitis B Infection) were classified as possible because they were negativity Hepatitis B virus surface antigen (HBsAg_), but the HBV-DNA (Desoxyribonucleic Acid) was not carried out. Therefore, these donors were soon excluded from the first and can be studied in the future.
Hepatitis C was 5.1 %; similarly, the prevalence was found in a study pilot in Burkina Faso at 5.2 % and high in Nigeria at 6.0 %,12,25 6.2 % in Gabon,26 8 % in Tanzania,27 and 8.5 % in South Ethiopia.28 Studies conducted in the Central Africa Republic,29 Burkina Faso,30 and South Gondar-Ethiopia31 have shown a low seroprevalence of HCV with 4.72 %, 4.40 % and 4.2 %, respectively. Other countries with low seroprevalence were Morocco with 1.51 %,32 Ethiopia with 1.6 %33 and Namibia with 0.1 %.10
According to the study, the prevalence of HIV in the blood donors population was 7 %. The proportion is considerably higher than other studies conducted elsewhere in Africa, like South Ethiopia, at 6.4 %,28 Egypt at 0.01 %,17 and Nigeria at 4.2 %34 and lowest when compared to Mozambique at 8.5 %,35 Equatorial Guinea at 7.83 %,36 South Africa at 9.8 %,37 Zambia at 15.9 %,38 Botswana at 22.9 %, Namibia at 9.1 %, and Swaziland at 26.1 %.39
The prevalence of Syphilis was 20 %, and when compared to other studies conducted in other African countries, Equatorial Guinea showed the highest prevalence of T.pallidum, 21.51 %36 and 40.5 % in Zambia.40 Three countries present a lower prevalence: Cameroon at 8.1 %,41 Ghana at 7.5 %,42 and Tanzania at 4.7 %.43 A study carried out in Namibia on Syphilis shows it to be more prevalent in women,10 which contradicts the findings of this study.
One of the main limitations of this study is that it was conducted with donations over five years. It is a retrospective blood donation card review, which might not include some variables. All test results did not give positive serological results during the window period, and it would be crucial for the study to cover other institutions by analysing the donors’ characteristics for several years with a much larger population for a more extended period. Many other studies should be done in Angola to assess the evolution of infections. The analysis of this study should be performed in several contexts for easy comparison with other studies. However, the data categorisation based on this study indicated the origin of most transfusion-transmitted infections.
We estimated a high seroprevalence of transfusion-transmitted infections among blood donors in Angola. Our study showed increased Hepatitis B prevalence and Syphilis and poor women participation. Identifying the seroprevalence of markers with positive results for Hepatitis B, Hepatitis C, HIV, Syphilis, and coinfection in blood donors is crucial to contribute to a strategy that can better control the transmission of these diseases, especially coinfection. The difficulty of obtaining safe blood has been well demonstrated, which raises the need for more accurate and statistical studies to establish better health policies for transfusion safety.
ConclusionThe present study shows a high seroprevalence of transfusion-transmitted infections among blood donors at Clínica Girassol in Luanda in the Angola Region. These trends of Hepatitis B, Hepatitis C, HIV and Syphilis prevalence suggest that it is crucial to be rigorous in implementing safety measures. Our study showed that it is essential to encourage more donations in voluntary donors for blood donation activities. Surveillance, control and prevention require continuous monitoring of the transmission of Hepatitis B, Hepatitis C, HIV and Syphilis, so it is imperative to improve the screening of donor selection criteria and the use of a combination of donor selection methods that provide safe blood in our country and thus contribute to the reduction of prevalence.
Author's contributionsAll the authors participated, read, and approved the final manuscript.
Cordeiro BL, Dias CC, Altamiro CP, and António Sarmento were involved in the conception and design of the study, interpretation of data, and drafting and revising of the manuscript. All authors read and approved the final manuscript.
Ethical considerationThis study was approved by the Institutional Ethics Committee (National Institute of Public Health of the Ministry of Health of Angola 25/2017) and (approval number 04/2018).
The research was conducted ethically, with all study procedures being performed in accordance with the requirements of the World Medical Association's Declaration of Helsinki.
Written informed consent was obtained from each participant and all donors signed an informed consent form for study participation and data publication.