There is an increasing use of daily chlorhexidine gluconate (CHG) bathing to decrease healthcare associated infections (HAI). Daily bathing of patients with CHG has been successfully used to prevent multidrug-resistant organisms (MDROs) HAI in intensive care units (ICU).
MethodsThis was a 12-month, single-center, open, cluster randomized trial, conducted at four ICUs of the University Hospital of Universidade Federal de São Paulo, Unifesp, Brazil. ICUs were randomized to either perform daily bathing of the patients with pH neutral soap and water – control units, or daily bathing with 2% CHG detergent solution – intervention units. We evaluated the incidence density rate of central line-associated bloodstream infection (CLABSI), ventilator-associated pneumonia (VAP), catheter associated urinary tract infection (CAUTI), Klebsiella pneumoniae carbapenemase (KPC)-producing enterobacteria HAI, and death in the intervention and control units.
ResultsA total of 1,640 admissions of 1,487 patients occurred during the study period (41.2% control group, and 58.8% intervention group). Incidence density rates of KPC-producing enterobacteria HAI were 5.01 and 2.25 infections/1000 patient-days in the control units and in the intervention units (p = 0.013) and mortality rates were 28.7% and 18.7% in the control units and in the intervention units (p<0.001), respectively. No difference between groups was observed in CLABSI incidence (p = 0.125), VAP incidence (p = 0.247) and CAUTI incidence (p = 0.435). No serious skin reactions were noted in either study group.
Daily 2% CHG detergent solution bathing is a feasible, low cost option for HAI prevention in ICU.
Multidrug-resistant organisms (MDROs), especially Gram-negative rods such as Klebsiella pneumoniae carbapenemase (KPC)-producing enterobacteria among other carbapenem-resistant Enterobacteriaceae (CRE), Pseudomonas spp, and Acinetobacter spp, have become the leading cause of healthcare associated infections (HAI) in many acute care facilities and Intensive Care Units (ICUs) in Sao Paulo city hospitals1,2 and globally.3,4 Infections caused by these organisms have limited effective antimicrobial options, are difficult to treat and are related to increased hospital length of stay, mortality rate, and high cost.3,4 HAI prevention, particularly those caused by MDROs is a complex process with multiple steps and interventions – care bundles, hand hygiene, active screening and isolation of patients with MDROs. Hospitalized patients, especially those in ICUs, with long stay and use of broad spectrum antimicrobials, are at high risk of skin colonization by health care-associated pathogens, with increased likelihood of subsequent infection.5 Chlorhexidine gluconate (CHG) is an antiseptic widely used in healthcare for reducing the patient's MDRO bioburden, with excellent safety profile.6,7 It has broad spectrum activity,8 and its antiseptic properties remain active on the skin for up to 24 hours.9,10 The use of a unit wide CHG daily bathing is a simple preventive measure that does not imply behavioral changes as much as hand hygiene and contact precautions.
Robert A. Weinstein was the first to study daily full body CHG bathing to prevent infections in ICUs.11 It has been used in several settings to control outbreaks and infections related to MDROs including methicilin-resistant Staphylococcus aureus (MRSA)12 vancomycin-resistant Enterococcus sp (VRE)11 and Gran-negative bacteria13–16 and fungi,17,18 but has no activity against mycobacteria and bacterial spores.19,20
Few studies have accessed the impact of daily 2% CHG detergent solution bathing on the incidence of HAI among ICU patients in Brazil. Abboud et al. in a pre- and post-intervention observational study, with CHG bathing as a part of a care bundle, found that the implemented measures were effective to reduce CRE colonization and central line-associated bloodstream infection (CLABSI) in a cardiac surgery ICU.21 In addition, CHG bathing was demonstrated to be economically advantageous.22 Sandri et al. have described, in nasal carriage of MRSA patients, a significant decrease in the incidence of nosocomial MRSA infection after 2% nasal mupirocin and CHG bathing in an ICU in relation to the pre-intervention period.23 Another quasi-experimental study, in a hematopoietic stem cell transplantation (HSCT) unit, with nine years of follow up, evaluated the impact of CHG bathing. VRE colonization and infection rates were reduced in the post-intervention period; however, MDR Gram-negative pathogens increased. At the post-intervention period, they could describe the emergence of a Pseudomonas aeruginosa clone.24
The aim of this study was to evaluate the impact of daily CHG solution bathing on the incidence of HAI among adult ICU patients at a tertiary-care teaching hospital in Brazil
MethodsSetting, design, intervention and outcomesThis was a single center, pragmatic, cluster randomized, non-blinded trial to evaluate the effect of unite wide daily 2% CHG detergent solution bathing on the incidence of HAI in four adult ICUs (one clinical-ICU with 17 patients, one neurosurgery-ICU with 9 patients, one trauma and non-trauma emergency surgery-ICU with 8 patients and one clinical/surgery-ICU with 9 patients), in a tertiary 754-bed teaching hospital - Hospital São Paulo, operated by the Federal University of São Paulo city, Brazil, in a 12-month period (06/01/14 to 05/31/15).
The four ICUs were randomized to either unit wide daily bathing of the patients with pH neutral soap (Sam Plus®) and water – control units, or daily bathing with 2% CHG detergent solution (Vic Pharma® or Riohex®) – intervention units, during the study period. Exclusion criteria were patients < 18 years of age, history of allergy including CHG allergy, extensive skin lesions - such as burnt lesions, and pregnancy. Neither the investigators nor the nursing staff were blinded to the two options of daily bathing.
Hospital and ICU admission and discharge date, or date of death and patient demographic, clinical and microbiological data were extracted from the hospital electronic chart. Active surveillance for HAI based on methods and definitions of the Brazilian National Health Surveillance Agency – (ANVISA) were performed in all units.25 ANVISA surveillance is based on current definitions used by the Centers for Disease Control and Prevention/National Healthcare Safety Network (CDC/NHSN),26 except VAP definitions that was still based on the previous CDC/NHSN definitions.27
The primary endpoints were overall HAI incidence, KPC-producing enterobacteria HAI incidence, and death. Secondary endpoint was adverse events related to CHG bathing, such as skin rashes, skin dryness and pruritus.
Prior to initiation of the study, a 2-month period (04/01/2014 to 05/31/2014) educational program on the proper techniques for 2% CHG detergent solution bathing, directed primarily toward nursing staff, was conducted. CHG bathing consisted of rinsing the entire body except the face – exposure to eyes and ears was avoided, with a cotton washcloth dampened with 2% CHG detergent solution (100 ml bottle - Vic Pharma® and Riohex®) and allow to air-dry without rinsing. To avoid skin dehydration due to CHG bathing, ICU patients received prophylactic skin hydrating lotion (NIVEA® Body Soft Milk or Almond oil LBS LABOROSA ®), at least 3 to 5 minutes after bathing. Those products were compatible with CHG. Soap and water bathing ICU patients received prophylactic hydrating lotion with Sam Plus® Body cream. Nursing staff were trained to monitor patients for skin reactions and report them to the investigators in a formulary.
Direct observation of bathing technique was conducted once a week in order to assure adherence to adequate technique. Compliance with 2% CHG detergent solution bathing was assessed by 50 direct observations. Time allowed to air-dry without rinsing ≤ 3 min occurred in 12 observations and face rinsing happened in three observations. Every week, the nursing staff were reinforced about bathing technics.
Statistical analysisCategorical variables are expressed as the count and percentage and were compared with chi-squared test. Continuous variables are expressed as mean and SD and were compared with T-test. When non-parametric tests (Mann-Whitney U) were used variables were expressed as median and quartiles (Q1 and Q3). P < 0.05 was considered statistically significant.
Ethical considerationsThe study was approved by the Research Ethics Committee of the Federal University of São Paulo review board (protocol no. 492.975 - CAAE: 23352713.1.0000.5505) with written informed consent obtained from each participant and/or their legal representative, as appropriate.
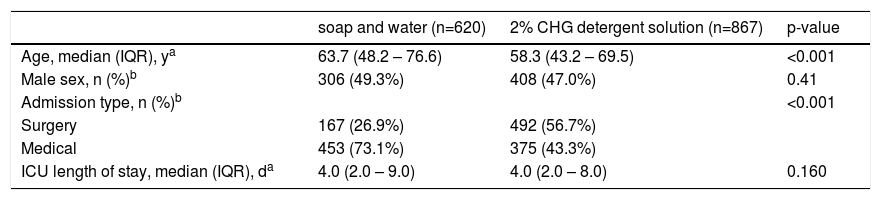
ResultsTwo ICUs were randomized for the intervention with 2% CHG detergent solution bathing and two ICUs were randomized for soap and water bathing. During the study period (June 1st 2004 to May 31st 20015), 1,524 patients were admitted to the four ICUs, 37 (2.2%) met the exclusion criteria, 12.2% of the patients had re-admission. When re-admission took place in the ICU with the same type of intervention, we just added the ICU length of stay of each admission, but when the re-admission was in a ICU with a different type of intervention, we count length of stay from each ICU. Therefore, the number of patients was different from the number of admissions. A total of 1,487 patients were included in the analysis, which corresponded to 1,640 admissions (620 patients admitted in the soap and water bathing ICUs and 867 in the 2% CHG detergent solution bathing ICUs) and 6,279 patient-days and 9,691 patient-days, respectively. Patients in the control ICUs were significantly older (mean age 63.7y) then patients in the intervention ICUs (mean age 58.3y), p < 0.001), and the number of surgical patients was significantly lower in the control ICUs (167 vs 492, p <0.001), sex and ICU length of stay in the two groups were not statistically different, as shown in Table 1.
Patient demographics and ICU length of stay.
| soap and water (n=620) | 2% CHG detergent solution (n=867) | p-value | |
|---|---|---|---|
| Age, median (IQR), ya | 63.7 (48.2 – 76.6) | 58.3 (43.2 – 69.5) | <0.001 |
| Male sex, n (%)b | 306 (49.3%) | 408 (47.0%) | 0.41 |
| Admission type, n (%)b | <0.001 | ||
| Surgery | 167 (26.9%) | 492 (56.7%) | |
| Medical | 453 (73.1%) | 375 (43.3%) | |
| ICU length of stay, median (IQR), da | 4.0 (2.0 – 9.0) | 4.0 (2.0 – 8.0) | 0.160 |
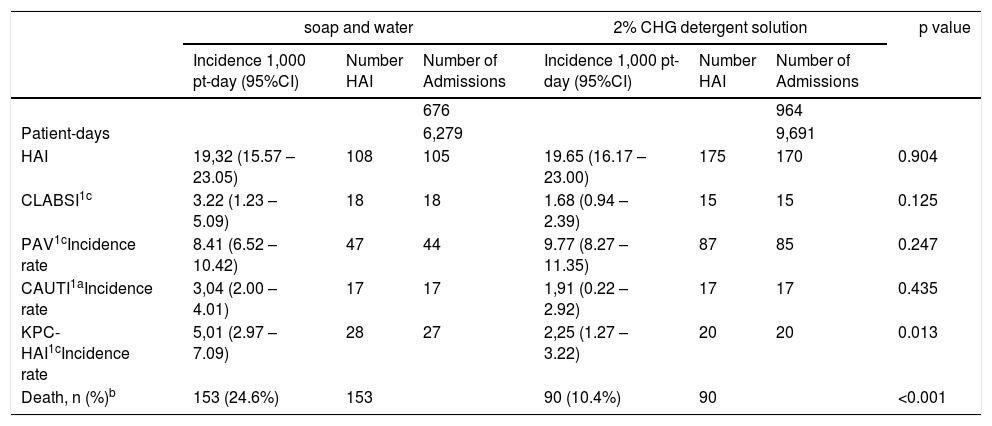
No difference between groups was detected in CLABSI incidence rate (p=0.125), VAP incidence rate (p=0.247) and CAUTI incidence rate (p=0.435). However, the incidence rate of KPC-producing enterobacteria HAI was significantly lower in the CHG bathing group when compared to the soap and water bathing group (5.01 vs 2.25, p=0.013). The mortality rate was also higher in the soap and water bathing group when compared to the CHG bathing group (28.7% vs 18.7%, p<0.001). CHG bathing was well tolerated. Skin reactions among patients assigned to CHG bathing was observed in 0.46% (4/867 patients) compared to 0.16% (1/620 patients) with soap and water bathing (p=0.61). All reported skin reactions were classified as mild to moderate in nature. as shown in Table 2.
Effect of Daily 2% CHG detergent solution on hospital-acquired infections (HAI), Klebsiella pneumoniae carbapenemase (KPC)-producing enterobacteria HAI and death.
| soap and water | 2% CHG detergent solution | p value | ||||||
|---|---|---|---|---|---|---|---|---|
| Incidence 1,000 pt-day (95%CI) | Number HAI | Number of Admissions | Incidence 1,000 pt-day (95%CI) | Number HAI | Number of Admissions | |||
| 676 | 964 | |||||||
| Patient-days | 6,279 | 9,691 | ||||||
| HAI | 19,32 (15.57 – 23.05) | 108 | 105 | 19.65 (16.17 – 23.00) | 175 | 170 | 0.904 | |
| CLABSI1c | 3.22 (1.23 – 5.09) | 18 | 18 | 1.68 (0.94 – 2.39) | 15 | 15 | 0.125 | |
| PAV1cIncidence rate | 8.41 (6.52 – 10.42) | 47 | 44 | 9.77 (8.27 – 11.35) | 87 | 85 | 0.247 | |
| CAUTI1aIncidence rate | 3,04 (2.00 – 4.01) | 17 | 17 | 1,91 (0.22 – 2.92) | 17 | 17 | 0.435 | |
| KPC-HAI1cIncidence rate | 5,01 (2.97 – 7.09) | 28 | 27 | 2,25 (1.27 – 3.22) | 20 | 20 | 0.013 | |
| Death, n (%)b | 153 (24.6%) | 153 | 90 (10.4%) | 90 | <0.001 | |||
This study presents one-year HAI incidence rates in four adult ICUs at a tertiary-care teaching hospital. Two ICUs were randomized to unit wide daily 2% CHG detergent solution bathing on the incidence of HAI. The incidence rates of CLABSI, PAV and CAUTI were not significantly different in the two groups. The incidence rates in all four ICUs were high, within the 50%, 75% and 75% percentiles, respectively, according to surveillance data of 48 adult ICUs at teaching hospitals in Sao Paulo state,26 and within the 90%, 75%, and 90% percentiles, respectively, according to CDC/NHSN surveillance of medical/ surgical ICUs of US major teaching hospitals.27 Therefore, a single intervention would be unlike to change this scenario. However, significantly reductions in the incidence rate of KPC-producing enterobacteria HAI and in the overall mortality rate were observed in the daily 2% CHG bathing units.
Bleasdale et al. in a crossover study have demonstrated reduction in the incidence of CLABSI, when comparing daily 2% CHG-impregnated washcloth bathing to soap and water bathing.28 Borer et al. could demonstrate 80% reduction on MDR-Acinetobacter baumannii skin colonization 24h after 4% CHG (liquid on a sponge) bathing on admission, and 85% reduction on the incidence of MDR-Acinetobacter baumannii BSI after the intervention with daily CHG bathing29 Climo et al., in a cluster-crossover study, described a 23% reduction of VRE and MRSA colonization and 28% reduction in BSIs after introducing 2% CHG-cloth bathing.17 In the REDUCE MRSA Study, 74 ICUs were submitted to either 1) MRSA screening and isolation, 2) screening, isolation, and decolonization of MRSA carriers with CHG bathing and nasal mupirocin, and 3) all patients decolonized with CHG cloth bathing and nasal mupirocin. The latter cluster - universal decolonization - was found to be associated with the greatest decrease in all-cause BSIs (44%; p = 0.001) and rates of MRSA clinical cultures (37%; p = 0.01).18 Huang et al. could demonstrate a 26% reduction in Gram-negative BSIs in the same trial.30 Noto et al., in a cluster-randomized crossover study, where patients were randomized to either CHG or nonantimicrobial bathing cloth for 10 weeks, then a 2-week washout period, and then crossed over to 10 weeks of the other bathing treatment, the use of CHG bathing cloth did not reduce the incidence of HAIs.8 Palloto et al. in a randomized controlled trial where 226 patients were enrolled in the intervention with daily 4% CGH bathing and 223 patients were enrolled to bathing with a standard soap, in ICU and post-operative cardiosurgical ICU, where the infectious diseases specialists were blinded to the intervention status, founded incidences of BSI and CLABSI significantly reduced in intervention patients (9.2 versus 22.6 infections/1000 patient-days, p = 0.027), but there was no difference in the mortality rate.31 In all these studies, there has been an improvement in a required, routine patient care activity which was associated with lower incidence of HAI, excepted in the study from Noto et al. Significant reductions in the incidence rate of KPC-producing enterobacteria HAI and the overall mortality rate in the intervention units were found in this study.
Our study has many limitations. First, it was a single center study and not a randomized clinical trial. It was not possible to control for many confounding variables, most important the kind of patient (clinical or surgical), use of invasive devices, and MDROs colonization at ICU admission. We did not audit every intervention, there was only a training period and a weekly basis visit. As it was not a blinded intervention, it has the potential for Hawthorne effect.
Our study also supports the recommendation that ICU patients over two months of age should be bathed with CHG on a daily basis to prevent CLABSIs.19 A secondary advantage of CHG bathing is to reduce blood culture contamination.32 However, chlorhexidine repeated exposure may decrease susceptibility with a potential emergency of resistance. Assessment of phenotypic resistance to CHG is still not well established and genotypic markers is challenging. Surveillance of clinical isolates should be considered in order to detect it. However, in many settings CHG bathing has been demonstrated to confer benefits.33–34.
Comparing the MDROs HAI prevention measures, such as hand hygiene and isolation and contact precautions, we believe that unit wide daily 2% CHG bathing is a feasible measure with less cost and less self-behavioral change, as it is incorporated into the nurse care routine.