The aim of this study was to clarify retrospectively the characteristics of children hospitalized for respiratory tract infection caused by macrolide-resistant Mycoplasma pneumoniae (M. pneumoniae).
MethodsChildren who were hospitalized for respiratory tract infection due to M. pneumoniae were enrolled in this study. The diagnosis of M. pneumoniae infection was made on the grounds of polymerase chain reaction results.
ResultsThirty-three children were hospitalized due to lower respiratory tract infection with M. pneumoniae. Of the 33 children, 31 (median age five years) were identified as being infected with macrolide-resistant M. pneumoniae (A2063G:30, A2064G:1) by sequence analysis. Of the 31 children infected with macrolide-resistant M. pneumoniae, 21 (68%) had received 14- or 15-membered macrolide antibiotics and four (13%) had received minocycline before hospitalization. During hospitalization, minocycline was administered to 16 (52%) of the 31 children infected with macrolide-resistant M. pneumoniae. Of the 20 children infected with macrolide-resistant M. pneumoniae under eight years of age, six (30%) were treated with minocycline during hospitalization. The difference in total febrile days between children receiving minocycline treatment before hospitalization and children not receiving minocycline treatment was three days.
ConclusionsThe majority of hospitalized children with respiratory tract infection due to macrolide-resistant M. pneumoniae infection was of preschool age and had received 14- or 15-membered macrolide antibiotics before hospitalization. Because macrolide-resistant M. pneumoniae is widespread in Japan, the administration of minocycline as a second-line antibiotic in children under eight years of age cannot be withheld when clinical symptoms do not improve with macrolide antibiotics.
Mycoplasma pneumoniae (M. pneumoniae) is a major cause of respiratory infection in school-age children and young adults. Because M. pneumoniae is sensitive to macrolides (14-membered ring: erythromycin, clarithromycin; 15-membered ring: azithromycin), M. pneumoniae illness is usually mild, and hospitalization is infrequently required. Since 2000, however, the emerging of macrolide-resistant M. pneumoniae has been reported in Japan, China, United States, and European countries, and recently, adults and children have been suffering from macrolide-resistant M. pneumoniae infections.1–12 Macrolide resistance rates in children with respiratory tract infection due to M. pneumoniae were reported to be 30.6% in Japan and 9.8% in France.4,6–8,10 The emergence of macrolide-resistant M. pneumoniae changes the clinical courses of children with respiratory tract infection.
Tetracyclines and fluoroquinolones can be used for the treatment of M. pneumoniae infections;13 however, these drugs are not recommended in children due to concerns about possible adverse effects. One of the adverse effects of tetracyclines is incorporation into tissues that are calcifying at the time of administration. Incorporation of tetracyclines into teeth, cartilage, and bone results in permanent discoloration varying from yellow to brown.14–18 Because the calcification of permanent teeth is not completed until 7–8 years of age, tetracyclines are not indicated for the treatment of common infection in children under eight years of age.19,20 In addition, fluoroquinolones have the potential to induce joint/cartilage toxicity in the pediatric population.21–23 Therefore, macrolides have been considered as first-line drugs for the treatment of M. pneumoniae infections in children.
The aim of this study was to clarify retrospectively the characteristics of children hospitalized for respiratory tract infection caused by macrolide-resistant M. pneumoniae in a regional hospital in Japan, where macrolide-resistant M. pneumonia infections are endemic. In addition, we analyzed the treatment options for macrolide-resistant M. pneumoniae infections in young children under eight years of age.
Patients and methodsPatientsChildren who were hospitalized in Eastern Yokohama Hospital between September 2010 and February 2012 for respiratory tract infection due to M. pneumoniae were enrolled in this study. All of them were diagnosed with pneumoniae or bronchitis on the basis of chest X-ray findings. The diagnosis of M. pneumoniae infection was made on the grounds of PCR results of nasopharyngeal or throat material collected with a swab. A febrile day was defined as a day in which the child's body temperature exceeded 38.0°C at least once. Clinical information was collected from medical records at Eastern Yokohama Hospital. Informed written consent for study participation was obtained from all of the children's parents or guardians. The study protocol was approved by the ethics committee of Eastern Yokohama Hospital (2012041) and conformed to the ethical guidelines of the Declaration of Helsinki.
PCR assayNasopharyngeal or throat swabs collected from patients were vigorously mixed with 500μL of PBS. Of the 500μL, 200μL was used for the extraction of M. pneumoniae DNA, which was accomplished using QIAamp DNA Blood Mini kit (QIAGEN, Hilden, Germany) according to the manufacturer's instructions. The extracted DNA was dissolved in 100μL of elution buffer and stored at −30°C. PCR assays were performed as described by Abele-Horn et al.24 In brief, PCR was performed in a 50μL reaction mixture containing 2.5U of Taq DNA polymerase (TaKaRa EX Taq, Takara Bio, Shiga, Japan) with 0.2μM primers and 10μL extracted DNA. The amplification was performed for 40 cycles (denaturation at 94°C for 30s, annealing at 55°C for 30s, and extension at 72°C for 45s) with a sense primer (MP-1: 5′-GAA GCT TAT GGT ACA GGT TGG-3′) and an antisense primer (MP-2: 5′-ATT ACC ATC CTT GTT GTA AGG-3′). PCR assay was performed in a GeneAmp PCR system 9700 (Applied Biosystems, Foster City, CA). The amplified PCR product was a 144-bp DNA fragment of the M. pneumoniae ATPase operon gene. A product of the predicted size (272bp) was observed after electrophoresis on a 2% agarose gel, ethidium bromide staining, and visualization under ultraviolet light.
Sequence analysis of the 23S rRNA geneTo identify the mutations of domain V of 23S rRNA, we performed nested PCR as previously described by Matsuoka et al.5 The first-round PCR product (927bp) was obtained using an outer sense primer (MN23SDVF; 5′-GCAGTGAAGAACGAGGGG-3′ [1758–1775]) and an outer antisense primer (MN23SDVR; 5′-GTCCTCGCTTCGGTCCTCTCG-3′ [2664–2684]). To detect the point mutation at 2063 and 2064 in domain V, we used an inner sense primer (MN23SF1937; 5′-ACTATAACGGTCCTAAGGTA-3′ [1918–1937]) and an inner antisense primer (MN23SR2128; 5′-ACCTATTCTCTACATGATAA-3′ [2108–2177]) for a second-round amplification, and a 210-bp PCR product was obtained. For the detection of point mutation at 2617 in domain, the inner sense primer (MN23SF2577; 5′-TACGTGAGTTGGGTTCAAA [2577–2595]) and antisense primer (MN23SR2664; 5′-GTCCTCGCTTCGGTCCTCTCG-3′ [2664–2684]) were used for a second round amplification, and a 108-bp PCR product was obtained. Nucleotide positions were designated on the basis of nucleotide sequences from M. pneumonia M129 (GenBank/EMBL accession number X68422). A product of the predicted size was observed after electrophoresis on a 2% agarose gel, ethidium bromide staining, and visualization under ultraviolet light. The DNA band was excised from the gel, and the DNA was purified using a QIAquick gel extraction and DNA purification kit (Qiagen, Hilden, Germany). All sequencing reactions were performed using the ABI Prism Big Dye Terminator Cycle Sequencing kit (Applied Biosystems).
ResultsPatients infected with macrolide-resistant M. pneumoniaeBetween September 2010 and February 2012, 33 children (male/female=16/17, age range 1–15 years, median age five years) hospitalized for respiratory tract infection were diagnosed with M. pneumoniae infection by PCR assay. The nucleotide sequence analysis of the 23S rRNA gene of M. pneumoniae showed that 31 children (94%) were infected with macrolide-resistant M. pneumoniae and the remaining two children (6%) were infected with wild-type M. pneumoniae. The mutations of A2063G and A2064G were detected in 30 children and in one child, respectively. The mutation of C2617G was not detected in any of the children.
Patients’ characteristics of the 31 children with macrolide-resistant M. pneumoniae at the first day of hospitalization are shown in Table 1. Febrile days before hospitalization ranged from three to 11, with a median of six. Of the 31 children infected with macrolide-resistant M. pneumoniae, 30 (97%) were treated with antibiotics as shown in Table 2. Of the 31 children infected with macrolide-resistant M. pneumoniae, 21 (68%) had a history of receiving erythromycin, clarithromycin, or azithromycin. The duration of administration of 14-membered macrolides ranged from two to five days, with a median of three days. Four of the children had a history of receiving minocycline before hospitalization. Of the four children administered with minocycline, three were under eight years of age (Table 2).
Patient characteristics of hospitalized children infected with macrolide-resistant Mycoplasma pneumonia.
| Male/female | 14/17 | |
| Age (yr.) | Median (range) | 5 (1–15) |
| Mean | 6.2 | |
| White blood cell counts (/μL) | Median (range) | 6520 (3410–15,570) |
| Mean | 6913 | |
| Serum C-reactive protein levels (mg/dL) | Median (range) | 2.5 (0.3–12.6) |
| Mean | 3.4 | |
| Number of days with fever before hospitalization | Median (range) | 6 (3–11) |
| Mean | 6.1 | |
| No. of patients prescribed 14- or 15-membered macrolide antibiotics before hospitalization | 21 (68%) | |
| Days of administration of 14-membered macrolide antibiotics before hospitalization (n=11) | Median (range) | 3 (2–5) |
| Mean | 3.3 | |
| No. of patients prescribed minocycline before hospitalization | 3 (10%) | |
| Days of administration of minocycline before hospitalization (n=3) | Median (range) | 3 (2–6) |
| Mean | 3.7 | |
| Mutations in 23S rRNA gene | A2063G | 30 |
| A2064G | 1 | |
| C2617G | 0 | |
Antimicrobial agents against macrolide-resistant Mycoplasma pneumoniae used before hospitalization.
| Under 8 years of age (n=20) | 8 years old or older (n=11) | |
|---|---|---|
| β-Lactam→clarithromycin | 3 | 2 |
| β-Lactam→azithromycin | 1 | 2 |
| Clarithromycin→azithromycin | 2 | 1 |
| Clarithromycin→minocycline | 2 | 0 |
| Tosufloxacin | 2 | 0 |
| β-Lactam | 2 | 2 |
| Azithromycin | 2 | 1 |
| Clarithromycin | 1 | 1 |
| Clarithromycin→azithromycin→tosufloxacin | 1 | 0 |
| β-Lactam→erythromycin | 1 | 0 |
| β-Lactam→josamycin | 1 | 0 |
| β-Lactam→minocycline | 1 | 0 |
| Norfloxacin→rokitamycine | 1 | 0 |
| Minocycline | 0 | 1 |
| No antibiotics | 0 | 1 |
The antibiotics administered during hospitalization to the children in this study are shown in Table 3. As initial antibiotics during hospitalization, minocycline was administered to 15 children, azithromycin to six children, tosufloxacin (quinolone) to four children, clarithromycin to two children, clindamycin to two children, and clarithromycin plus clindamycin to one child. One child did not receive any antimicrobial agents during hospitalization. Of the 11 children who were aged eight or more, 10 (91%) were treated with minocycline as the initial antibiotic during hospitalization. In four of the 20 children under eight years of age, the initial antibiotics were switched to other antimicrobial agents during hospitalization. As second antibiotics during hospitalization, minocycline was administered to one child and tosufloxacin to three children. The initial antibiotics were not changed in any children who were over 8 years of age.
Antimicrobial agents against macrolide-resistant Mycoplasma pneumoniae duration hospitalization.
| Under 8 years of age (n=20) | 8 years old or older (n=11) | |
|---|---|---|
| Minocycline | 5 | 10 |
| Tosufloxacin | 4 | 0 |
| Azithromycin | 2 | 1 |
| Clarithromycin | 2 | 0 |
| Clindamycin hydrochloride | 2 | 0 |
| Azithromycin→tosufloxacin | 2 | 0 |
| Azithromycin→minocycline | 1 | 0 |
| Clindamycin hydrochloride+clarithromycin→tosufloxacin | 1 | 0 |
| No antibiotics | 1 | 0 |
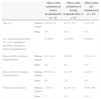
To evaluate the effectiveness of minocycline, we compared the number of febrile days among the children administered minocycline before hospitalization, the children administered minocycline during hospitalization (excepting the children to whom minocycline was administered before hospitalization), and the children who did not receive minocycline (Table 4). There was no difference in the number of days with fever before hospitalization (median from 6 to 6.5 days) among the three groups. The number of days with fever during hospitalization ranged from 0 to 1 (median 0.5 day) among children who received minocycline before hospitalization. In the children who received minocycline during hospitalization, the number of days with fever during hospitalization ranged from one to five (median two days). On the other hand, the number of days with fever during hospitalization ranged from zero to seven (median four days) in children who did not receive minocycline. Similarly, the median of total number of days with fever in children who received minocycline was 3-day lower than that in children who had not received minocycline. These findings suggest that minocycline contributed to a 3-day-earlier resolution of fever in children infected with macrolide-resistant M. pneumoniae.
Days with fever of patients infected with macrolide-resistant Mycoplasma pneumonia.
| Minocycline administered before hospitalization (n=4) | Minocycline administered during hospitalization (n=12) | Minocycline not administered (n=15) | ||
|---|---|---|---|---|
| Age (yr.) | Median (range) | 4.5 (4–11) | 8.5 (3–15) | 4 (1–11) |
| Mean | 6.0 | 8.6 | 4.3 | |
| No. of patients prescribed 14- or 15-membered macrolide antibiotics before hospitalization | 2 (50%) | 9 (75%) | 9 (60%) | |
| Days with fever before hospitalization | Median (range) | 6.5 (5–8) | 6 (5–9) | 6 (3–11) |
| Mean | 6.5 | 6.1 | 6.1 | |
| Days with fever during hospitalization | Median (range) | 0.5 (0–1) | 2 (1–5) | 4 (0–7) |
| Mean | 0.5 | 2.6 | 4.3 | |
| Total days with fever | Median (range) | 7 (6–8) | 8.5 (6–11) | 10 (6–16) |
| Mean | 7 | 8.5 | 10.3 | |
All 31 children infected with macrolide-resistant M. pneumoniae had cough and dyspnea. The pulsed oxygen saturation ranged from 86% to 98% (median 93%). Of the 31 children, 21 (65%) required oxygen supplementation due to hypoxia during hospitalization. None of them required mechanical ventilation. All of them were cured without consequences.
DiscussionOf the 33 hospitalized children with respiratory tract infection caused by M. pneumonia, 31 (94%) were infected with macrolide-resistant M. pneumonia. Usually, the peak incidence of M. pneumoniae occurs in school-aged children and young adults. In this study, however, the median age of the 31 children infected with macrolide-resistant M. pneumoniae was five years of age. Of these 31 children, 21 (68%) had already been treated with 14- or 15-membered macrolide antibiotics before admission. In addition, fever had persisted for six days (median) before admission in the 31 children infected with macrolide-resistant M. pneumoniae. These findings suggest that the majority of hospitalized children suffering from M. pneumoniae infection in Japan are infected with macrolide-resistant strains and pre-school children are likely to show persistent clinical symptoms such as high fever and cough even if macrolide antibiotics are administered in case of macrolide-resistant M. pneumoniae.
The total number of days with fever in children infected with macrolide-resistant M. pneumoniae in this study was comparable with that reported in a previous study, which showed a median of 10 days.25 The authors of the previous study reported a median of 3.5 days with fever during 14-membered ring macrolides administration in patients with macrolide-resistant M. pneumoniae infection, indicating that four days of administration of macrolides is sufficient to control macrolide-resistant M. pneumoniae infection. However, the present study showed that children infected with macrolide-resistant M. pneumoniae had already received 14-membered macrolide antibiotics for three days (median) before hospitalization and high fever persisted during hospitalization even with continuing use of macrolides. These findings suggest that it takes four days or more for the treatment with macrolide antibiotics to control macrolide-resistant M. pneumoniae infection.
There are currently two choices for pediatricians to treat hospitalized children infected with macrolide-resistant M. pneumoniae. One is to continue to use macrolide antibiotics and waiting for the resolution of clinical symptoms, because fatal outcome of M. pneumoniae infection is rare. The other choice is to discontinue macrolide antibiotics and try to use tetracyclines or fluoroquinolones. In this study,23 31 (74%) children infected with macrolide-resistant M. pneumoniae received minocycline or tosufloxacin during hospitalization. These findings indicate that the majority of pediatricians prefer to discontinue macrolide antibiotics during hospitalization. Minocycline has been reported to be effective in macrolide-resistant M. pneumoniae infection.4,9,11,26 The present study showed that the difference in the number of days with fever during hospitalization between children receiving pre-hospitalization minocycline treatment and children not receiving minocycline treatment was of three days. Although this was a retrospective study and not a controlled study, these findings indicate that the early treatment with minocycline was effective against macrolide-resistant M. pneumoniae infection in children. In the case of the young child with respiratory failure described above, the administration of minocycline was required to improve clinical symptoms.
Of the 16 children treated with minocycline before or during hospitalization, two (four years old, 11 years old) received minocycline as first-line antimicrobial agent, while the remaining children received minocycline as second-line microbial agent through their clinical courses. Of the 11 children who were aged eight years or older, 10 (91%) were treated with minocycline during hospitalization. Although minocycline is contraindicated or not indicated in children under eight years of age, six (38%) of the 16 children treated with minocycline were under eight years in this study. The prevalence of tetracycline and minocycline tooth staining was reported to be approximately 3–6%.19 Moreover, there are some reports of minocycline-derivative tooth staining in adults.14,15,27,28 However, the association between dose and tooth staining is controversial.19 In contrast, a previous study reported that there was no significant difference in dental staining and defects between children under eight years of age treated with oral minocycline for brucellosis and matched controls.29 Normally, minocycline should not be administered as a first-line antimicrobial agent against M. pneumoniae infection in young children. When minocycline is administered in children under eight years of age after considering the benefits and risks, it is essential to inform parents that minocycline has the potential to stain tooth.
Seven children treated with non-minocycline received tosufloxacin, which is a quinolone antibiotic. In 2010, tosufloxacin was approved for children by the Japanese Health and Labor Ministry. The antimicrobial mechanism of quinolone is different from that of macrolides,1,6,30–32 and tosufloxacin is considered by some clinicians to be effective against macrolide-resistant M. pneumoniae infection. However, further studies are required to determine the effectiveness of tosufloxacin in macrolide-resistant M. pneumoniae infection.
In conclusion, preschool children with lower respiratory tract diseases due to macrolide-resistant M. pneumoniae infection tend to be hospitalized. The majority of the children with macrolide-resistant M. pneumoniae infection in our study had received 14- or 15-membered macrolide antibiotics before hospitalization. The treatment with minocycline contributed to the early resolution of clinical symptoms. Although minocycline should not be used as first-line antimicrobial agent in children under eight years of age, minocycline could be allowed to be administered if the respiratory condition worsens during the administration of macrolide antibiotics.
Ethical approvalThe study protocol was approved by the ethics committee of Eastern Yokohama Hospital (2012041).
Authors’ contributionsHK contributed to the design of this study and drafted this manuscript. TT, AI, TS and TF participated in data collection and critical revision of the manuscript.
Conflicts of interestThe authors declare no conflicts of interest.