Candida albicans utilizes arachidonic acid (AA) released during the course of infection (Candidiasis) from phospholipids of infected host cell membranes and synthesizes extracellular prostaglandin(s) which play an important role in hyphae formation and host cell damage. C. albicans biofilms secrete significantly more prostaglandin(s) and evidence suggests that Candida biofilms have dramatically reduced susceptibility to majority of antifungal drugs. AA influences the saturation level of lipids and fluidity of yeast cell membranes. Therefore the aim of this study was to evaluate the effect of AA alone or in combination with antifungal agents on biofilm formation and production of prostaglandin (PGE2) in C. albicans, C. parapsilosis, C. glabrata, C. tropicalis, and C. albicans amphotericin B resistant strain (AmBR). Maximum biofilm formation was found to be in the case of C. albicans compared to C. non-albicans species. However, among the non-albicans species C. tropicalis exhibited highest biofilm formation. Treatment with AA in combination with subinhibitory concentrations of fluconazole and terbinafine separately exhibited significant (p<0.05) reduction in biofilm formation against C. glabrata, C. parapsilosis, C. tropicalis and AmBR as compared to their individual effect. Further, these two antifungal agents in combination with AA caused an increase in production of prostaglandin from fungal cell itself which was significant (p<0.05) in case of all the strains tested.
The pathogenic species of the genus Candida have gained recognition as nosocomial agents in recent years which may be attributed to specific risk factors associated with modern therapeutics like immunosuppressive agents, cytotoxic drugs, and broad-spectrum antibiotics that suppress the normal bacterial microbiota. Likewise, use of various medical implants may result in Candida biofilm formation that often leads to aggravation of infection. Evidence suggests that Candida biofilms have dramatically reduced susceptibility to clinically used antifungal drugs like fluconazole and amphotericin B. Biofilm formation capacity of C. albicans and its role in virulence and antifungal resistance has been studied in detail.1Candida non-albicans species like C. parapsilosis and C. tropicalis have also been found as biofilm forming pathogens in recent past.2,3
Candida albicans has been reported to be responsible for the release of arachidonic acid (AA) from the host cells during infections4,5 which may modulate the cell growth, morphogenesis and invasiveness of causal agent by several modes. AA is a precursor for the production of eicosanoids which play an important role in morphogenesis and biofilm formation. Prostaglandin E2 (PGE2) is a primary product of AA metabolism in most of the eukaryotic cells that has also been reported in pathogenic fungi as well.6,7 Enhanced prostaglandin production during fungal infections could be one of the important factors in promoting colonization as well as chronic infections. The shift in host immune response toward increased colonization and chronic infections is due to PGE2, which has ability to elicit both pro- and anti-inflammatory responses depending upon the host cells.8,9
Exogenous AA has been reported to increase PGE2 level significantly in C. albicans whereas the behavior of Candida non-albicans species and resistant strains in presence of AA is not much studied till now.8–10 The studies related to determining the level of PGE2 in C. albicans and C. non-albicans species in presence of AA may help in understanding the biofilm forming capacity. The purpose of the present study was to compare the biofilm formation capacity and the level of prostaglandin secretion of the C. albicans and Candida non-albicans species in the presence of AA and known antifungal agents. The study also explores the pattern and amount of biofilm formation and the amount of prostaglandin secreted by a laboratory induced amphotericin B resistant C. albicans strain (AmBR).11
Materials and methodsCandida strains usedFive species of Candida were used in this study. Out of these four species viz. C. albicans (ATCC-10231), C. glabrata (ATCC-MYA2950), C. parapsilosis (ATCC-22019), and C. tropicalis (ATCC-750) were purchased from ATCC (American type cell culture) while the fifth was an amphotericin B resistant strain of C. albicans (AmBR) developed earlier in our laboratory.11
Media used and growth conditionsAll the test strains maintained on Sabouraud dextrose agar were grown in yeast extract peptone and dextrose (YPD) medium (Difco BD, USA) for 24h at 28°C in an incubator shaker (180rpm). The yeast cells were harvested, washed twice with 0.15M phosphate-buffered saline (PBS, pH 7.4) and suspended in 10ml of same buffer.
Antifungal susceptibility in vitroThe minimal inhibitory concentrations (MICs) of fluconazole (Sigma Aldrich, USA) and terbinafine (Biomole, USA) against all the five strains (as above) were determined by broth micro-dilution method as per guidelines of CSLI (formerly NCCLS)12 using RPMI 1640 (Sigma Aldrich, USA) buffered with MOPS (Sigma Chemical Co.) in 96-well tissue culture plates with positive and negative growth as well as drug controls. The test antifungals terbinafine and fluconazole were dissolved in DMSO (Merck, Germany) at a concentration of 2mg/ml and 0.64mg/ml respectively, diluted twofold and tested against 2×103cells per ml of the test fungi. The micro-titer plates were incubated at 35°C in a moist, dark chamber and MICs were recorded spectrophotometrically (Spectra Max, Molecular devices, USA) at 490nm after 48h incubation.
MICs of fluconazole and terbinafine were determined against biofilm formation by using the standard CLSI micro dilution protocol M27-A.12–14 Briefly, 1–5×103cells/ml were incubated in RPMI 1640 buffered with MOPS under shaking condition at 37°C for 90min and the plates were washed three times with PBS to remove non-adherent cells. The test antifungals fluconazole (32–0.25μg/ml) and terbinafine (100–0.39μg/ml) were diluted twofold in a replica plate and the whole contents transferred to the plates having the culture of biofilms and incubated for 48h at 37°C. Subsequently the plates containing Candida biofilms were washed thrice with PBS and 50μl of XTT salt solution (1mg/ml in PBS) and 4μl of menadione solution (1mM in acetone; Sigma) added to each well, incubated at 37°C for 5h, and XTT formazan measured in the supernatant medium at 490nm by using a spectrophotometer (Molecular Devices, USA) where ≥80% decrease in formazan (OD at 490nm) was taken as MIC. The experiment was done in duplicate and repeated three times on different occasions.
Biofilm formationAll the strains were subjected to biofilm formation under the following growth conditions separately: YNB with 50mM glucose, YNB with 50mM Glucose supplemented with 500μM AA (Sigma Aldrich, USA), YNB with 50mM glucose added subinhibitory concentration (1/2MIC) of fluconazole, YNB with 50mM glucose added 1/2MIC terbinafine, YNB and 50mM glucose supplemented with 1/2MIC fluconazole and AA and YNB supplemented with 50mM glucose, 1/2MIC terbinafine and AA. Here it may be mentioned that subinhibitory concentration (1/2MIC) of both the drugs fluconazole and terbinafine against biofilm were used (Table 1). Biofilm formation was carried out in 96 well culture plates.15 Two-hundred μl fungal suspensions (1×106cells) of all the test strains in the above medium were poured in 96 well tissue culture plates separately, kept under mild shaking condition for 90min and subsequently the non-adherent cells were washed out with PBS. Then 200μl media containing appropriate growth conditions as above was added in each well and incubated at 37°C for 48h.16,17
Minimal inhibitory concentration (MIC) of antifungal agents against different species and strains of Candida.
| Strains | Terbinafine (MIC in μg/ml) | Fluconazole (MIC in μg/ml) | ||||||
|---|---|---|---|---|---|---|---|---|
| Planktonic | Biofilm | Planktonic | Biofilm | |||||
| MIC | 1/2MIC | MIC | 1/2MIC | MIC | 1/2MIC | MIC | 1/2MIC | |
| C. albicans | 18.3 | 9.2 | >100 | 100 | 0.36 | 0.18 | 4.0 | 2.0 |
| AmBR | 0.23 | 0.12 | 50.0 | 25.0 | 0.61 | 0.30 | 32.0 | 16.0 |
| C. glabrata | 72.0 | 36.0 | >100 | 100 | 3.31 | 1.65 | 32.0 | 16.0 |
| C. parapsilosis | 0.29 | 0.14 | 50.0 | 25 | 0.74 | 0.37 | 2.0 | 1.0 |
| C. tropicalis | 68.0 | 34.0 | >100 | 100 | 0.19 | 0.09 | 0.25 | 0.12 |
XTT reduction assay of biofilm.
Quantitation of Candida biofilms was performed by using 2,3-bis (2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT; Sigma Aldrich, USA) reduction assay where the water-soluble formazan product was measured at 490nm.17,18 Following the biofilm formation phase, culture plates containing Candida biofilms were washed thrice with PBS and 50μl of XTT salt solution (1mg/ml in PBS) and 4μl of menadione solution (1mM in acetone; Sigma) added to each well. The plates were incubated at 37°C for 5h and XTT formazan measured in the supernatant medium at 490nm by using a spectrophotometer (Molecular Devices, USA). The experiment was done in duplicate and repeated three times on different occasions.
Fluorescence microscopyTo study the biofilm formation fluorescence microscopy was performed in six well tissue culture plates and the images were obtained on Leica DM2500 Fluorescence microscope. Briefly, biofilms of above strains were grown in six well tissue culture plates in RPMI 1640 with l-glutamine and 0.165M MOPS under different conditions of media as mentioned above. After incubation each well was washed with DPBS, stained with 100mg/ml fluorescein diacetate (FDA; Sigma Aldrich, USA) and 50mg/ml propidium iodide (PI; Sigma Aldrich, USA) for 3h and viewed under microscope.19
Prostaglandin E2 quantitationSupernatant obtained (after 48h) from the biofilm culture of all the strains under different growth conditions as mentioned above were subjected to PGE2 estimation. Prostaglandin E2 Express EIA kit (Cayman Chemicals) was used according to manufacturer's instructions. Briefly, cell culture supernatant was used directly for prostaglandin estimation and appropriate standards and controls were run simultaneously to obtain a standard curve. Each sample was assayed in triplicate and the experiments were repeated three times on different occasions.10 Sample concentrations were calculated on the basis of standard curve (plotted against standards versus PGE2 concentration) and the data represented is the average of three experiments.
Statistical analysisAll experiments were performed in triplicate unless stated otherwise. The t-test was performed to determine the significance of the data sets.
Results and discussionCandidiasis is one of the common fungal infections in humans and C. albicans being endogenous has been considered a major causative agent but in recent past, but Candida non-albicans species have also marked their presence as frequent pathogens. The major clinical impact generated by Candida species is their ability to form biofilms which results in increased resistance to antifungal therapy and the ability of yeast cells within the biofilms to withstand host immune defences. Over and above it is interesting to note that there are species-specific variations in Candida biofilm morphology and formation capacity.1–3 Considering this, two antifungals, fluconazole and terbinafine were included in this study knowing the fact that the two are chemically different and yet block the synthesis of ergosterol.
Antifungal susceptibility against fluconazole and terbinafineMICs were determined by CLSI method12 for fluconazole and terbinafine against both planktonic and biofilm cells of C. albicans, C. glabrata, C. parapsilosis, C. tropicalis and AmBR. In the present study fluconazole (0.19–32μg/ml) exhibited overall better spectrum of efficacy as compared to terbinafine (0.23–>100μg/ml) against planktonic cells as well as biofilm cells (Table 1). However terbinafine exhibited an edge over fluconazole in case of AmBR and C. parapsilosis (Table 1).
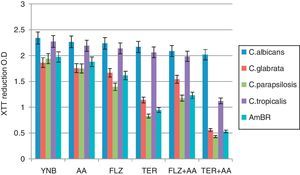
Biofilm formationIn the present study, biofilm formation was studied in C. albicans and Candida non-albicans species (C. glabrata, C. tropicalis, and C. parapsilosis) and a laboratory developed amphotericin B resistant (AmBR) strain of C. albicans.11 Polystyrene 96-well cell culture plates were used for biofilm formation in YNB media, which was measured by XTT reduction assay (Fig. 1). It is known that biofilms exhibit resistance against antifungals compared to planktonic cells; thus, the sub-inhibitory concentrations (1/2MIC, Table 1) of antifungal agents may have several important implications on planktonic cells destined to form biofilms.14,18,19 The influence of different treatments (growth conditions) was compared with the formation of biofilms in YNB alone which was considered as 100% for each of the five strains tested. In the present study YNB supplemented with AA (500μM) did not affect the biofilm formation so the results indicate that 500μM AA has no influence on the viability of the biofilms. The subinhibitory concentration of fluconazole caused reduced biofilm formation in case of C. parapsilosis (71.9%±2.9, p<0.05) and the resistant strain AmBR (81.9%±3.2, p<0.05). The biofilms of C. albicans as well as C. non-albicans species are known to exert resistance against many antifungal agents including fluconazole,2,3 which has also been reported to reduce biofilm formation in clinically isolated fluconazole susceptible and resistant strains of C. albicans.17 However, in the present study a reduction in biofilm formation was evident in case of AmBR only (Fig. 1 and Table 2). The subinhibitory concentration of terbinafine caused significantly (p<0.0005) reduced biofilm formation in case of C. parapsilosis (42.7%±1.6,), AmBR (47.8%±1.6) and C. glabrata (61.2%±1.4) while in case of remaining two strains non-significant reduction was noticed (p>0.05, Table 2).
XTT reduction assay of biofilm.
Biofilm formation by Candida albicans, non-albicans, and AmBR under the influence of different media treatment was estimated by XTT assay. Biofilm formation in YNB alone was considered as control. Further 1/2MIC of fluconazole (FLZ) and terbinafine (TER) were supplemented in medium individually or in combination with arachidonic acid (AA). Results exhibit that FLZ and TER affects biofilm formation but it was more pronounced when tested in combination with AA in comparison to control (p<0.005) and least biofilm formation was observed in case of C. parapsilosis followed by AmBR and C. glabrata. However, this effect was more pronounced with TER (p<0.005) in comparison to FLZ.
Biofilm formation (in %) under the influence of different media treatment.
| Strains | % growth of biofilm under the influence of different media | ||||
|---|---|---|---|---|---|
| YNBa+AA | YNBa+1/2FLZ | YNBa+1/2TER | YNBa+AA+1/2FLZ | YNBa+AA+1/2TER | |
| C. albicans | 95.9±1.2 | 96.4±1.02 | 93.6±1.4 | 91.0±.2.4 | 86.1±1.8 |
| AmBR | 94.1±1.7 | 80.8±3.2 | 47.2±1.6 | 61.4±1.9 | 26.5±1.2 |
| C. glabrata | 93.6±1.4 | 91.9±2.9 | 62.3±1.4 | 82.6±2.3 | 31.6±1.4 |
| C. parapsilosis | 90.3±1.8 | 71.8±2.9 | 42.7±1.6 | 60.6±2.2 | 23.0±1.3 |
| C. tropicalis | 99.1±1.1 | 96.6±1.2 | 93.1±1.4 | 88.6±2.3 | 48.9±2.4 |
It is encouraging to note that both the antifungals when tested in combination with AA resulted in further inhibition in biofilm formation as compared to either of the two agents (antifungal and AA) tested alone. Our results exhibit that fluconazole and terbinafine when tested in combination with AA caused least biofilm formation in case of C. parapsilosis followed by AmBR and C. glabrata. However, this effect was more pronounced (p<0.0005) with terbinafine in C. parapsilosis (23%±1.3), AmBR (26.5%±1.2) and in case of C. glabrata it was 31.6%±1.4 (Fig. 1 and Table 2). In addition to this terbinafine with AA also reduced biofilm formation of C. tropicalis indicating that terbinafine with AA was the best combination in reducing biofilm formation against test strains as well as resistant strain AmBR (Table 2).
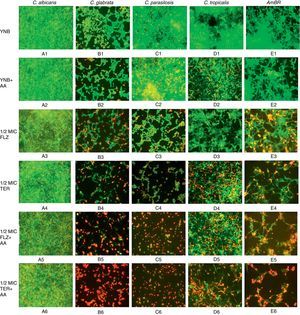
Fluorescence microscopy for biofilm evaluationThe biofilm cells of the test Candida strains were investigated by staining with FDA and PI and viewed under fluorescence microscope. The live and dead cells of biofilms were distinguished by green (FDA) and red (PI) fluorescence respectively indicating the presence of both viable and dead cells in varied amounts with regard to various treatments. The biofilm formation of all the test strains in YNB alone (Fig. 2A1–E1) were used as respective control where green fluorescent images were observed suggesting the viability of biofilm cells. Treatment of biofilms with various test agents like AA alone, 1/2MIC fluconazole, 1/2MIC terbinafine and 1/2MIC of antifungals combination with AA caused varied fluorescence (Fig. 2). The subinhibitory concentration (1/2MIC) of fluconazole and terbinafine also exhibited some changes (Fig. 2A3–E3 and A4–E4) in fluorescence as compared to their respective controls which is in agreement with the results of XTT assay (Fig. 1). As expected the subinhibitory concentration of fluconazole and terbinafine supplemented with AA resulted in significant suppression of biofilm formation (Fig. 2A5–E5 and A6–E6). Fluorescence microscopy of test strains with different treatments exhibited that 1/2MIC of antifungals in combination with AA affect the biofilm formation of all the test strains, which was greater in case of terbinafine as compared to fluconazole (Fig. 2).
Fluorescence microscopy of biofilm: fluorescence microscopy comparison of biofilms grown in presence and absence of arachidonic acid (AA) treated with subinhibitory concentration of fluconazole and terbinafine exhibited that least biofilm formation was observed in case of C. glabrata (Figs. B5 and B6), C. parapsilosis (Figs. C5 and C6) and AmBR (Figs. E5 and E6) compared to that in case of C. albicans (Figs. A5 and A6). The test strains with different treatments exhibited that 1/2MIC of antifungals with AA affect the biofilm formation of all test strains and among all treatments 1/2MIC of terbinafine with AA has greater effect than 1/2MIC fluconazole with AA. This result also suggests that terbinafine increases the susceptibility with AA as well as reduces the biofilm formation.
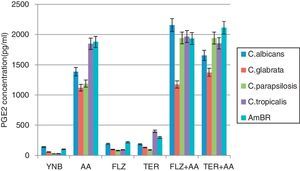
The planktonic and biofilm forms of C. albicans have been reported to produce eicosanoids particularly prostaglandin itself and/or in the presence of AA which is greater in biofilms.10 Prostaglandins have been known to be potential molecules to mediate cross talk between host and pathogen resulting in down-regulation of host immunological responses. Prostaglandins also play an important role in the persistence of fungal pathogen in immunocompetent hosts resulting in chronic infections. As stated above enhanced prostaglandin production has been observed in C. albicans biofilm10 whereas its production in biofilms produced by Candida non-albicans species and drug resistant strain has not yet been studied. The amount of PGE2 secreted by the test strains under control condition (YNB alone) for C. albicans (141pg/ml, ±6.2),C. glabrata (57.3pg/ml, ±1.3), C. parapsilosis (28.1pg/ml, ±1.1), C. tropicalis (29.6pg/ml, ±2.4) and AmBR (100.2pg/ml, ±2.1) was considered as basal concentration and the amount of PGE2 was calculated after subtracting the basal concentration (Fig. 3). An increase in PGE2 synthesis was noted for C. albicans (1387±15.0pg/ml), AmBR (1878±11.0pg/ml), C. parapsilosis (1189.3±13.2pg/ml), C. glabrata (1123.0±15.5) and C. tropicalis (1846±15.3pg/ml) when grown in YNB supplemented with AA (p<0.005). These results suggest that AA could have been incorporated into the phospholipids of yeasts, influencing the saturation level and fluidity of yeast cell membranes. Influence of AA on cell membrane phospholipids unsaturation and ergosterol content has been reported recently.10,20 The reduced ergosterol content of amphotericin B resistant strain11 could have been saturated with phospholipids incorporated by AA. Therefore increased amount of membrane phospholipids enables AmBR to produce more PGE2 than its parent strain.
PGE2 level estimation of C. albicans and non-albicans biofilms.
The level of PGE2 produced by Candida albicans and non-albicans biofilm in different media treatment was estimated after 48h incubation. Synthesis in YNB medium was used as control, further supplemented with arachidonic acid (AA), 1/2MIC of fluconazole (FLZ) and terbinafine (TER) individually. Result exhibited that PGE2 formation was increased in the presence of 1/2MIC antifungals and it is more pronounced in C. tropicalis and AmBR against TER. The level of PGE2 in all test strains including AmBR, was found to be enhanced under medium of YNB supplemented with AA, 1/2MIC FLZ (p<0.0005) and AA, 1/2MIC TER (p<0.0005). PGE2 level was estimated by ELISA method (Cayman Kit). Result suggested that AA enhanced PGE2 production in the presence of subinhibitory concentration of antifungals (1/2MIC).
In this experiment the results show that prostaglandin production is enhanced when AA was supplemented with subinhibitory concentration of fluconazole, C. albicans exhibited 2157.3±26.1pg/ml PGE2 followed by C. tropicalis (1966.6±14.0pg/ml), C. parapsilosis (1944±14.5pg/ml), AmBR (1935±16.5pg/ml) and C. glabrata (1174.3±9.2pg/ml) (Fig. 3 and Table 3). PGE2 production in the presence of AA with subinhibitory concentration (1/2MIC) of terbinafine was more pronounced (p<0.0005; Fig. 3 and Table 3) against AmBR (2114±12.2pg/ml) and C. glabrata (1374.3±16.5pg/ml). The present results open a new dimension in prostaglandin research as Candida non-albicans and C. albicans drug-resistant strain (AmBR) exhibited a notable difference in production of PGE2 individually.
PGE2 level (pg/ml) estimated under the influence of arachidonic acid (AA).
| Strains | PGE2 level (pg/ml) in different conditions | ||
|---|---|---|---|
| YNB+AAa | YNB+AA+1/2MIC FLZ | YNB+AA+1/2MIC TER | |
| C. albicans | 1387±15.0 | 2157.3±26.1 (p<0.0001) | 1655.3±14.1 (p<0.0001) |
| AmBR | 1878±11.0 | 1935±16.5 (p<0.005) | 2114±12.2 (p<0.0001) |
| C. glabrata | 1123.0±15.5 | 1174.3±9.2 (p<0.05) | 1374.3±16.5 (p<0.0001) |
| C. parapsilosis | 1189.3±13.2 | 1944±14.5 (p<0.0001) | 1942.3±2.5 (p<0.0001) |
| C. tropicalis | 1846±15.3 | 1966.6±14.0 (p<0.005) | 1858±9.1 (p<0.1) |
The variation in PGE2 production in biofilms of the different test strains could be indicative of their persistence in host tissues. The level of PGE2 in all test strains, including AmBR, was found to be enhanced under medium of YNB with AA+1/2MIC fluconazole and with AA+1/2MIC terbinafine (p<0.0005, Fig. 3). This can be explained by the fact that AA increases susceptibility of C. albicans as well as Candida non-albicans species against antifungal agents and may affect the prostaglandin production as well. Thus, it is evident from the results that the exposure to antifungal agents enhances the productions of PGE2 to a greater extent in biofilms which may be responsible for development of resistance against antifungal agents.
ConclusionThe main general conclusion from this study is that the effect of subinhibitory concentrations of antifungal agents on biofilm formation is dependent on the type of antifungal agent and fungal strain used. Fluconazole and terbinafine when tested in combination with AA caused least biofilm formation in case of C. parapsilosis followed by AmBR and C. glabrata. However, this effect was more pronounced with terbinafine in terms of antifungal activity as well as reduced biofilm formation. Our results further suggest that AA could have been incorporated into the phospholipids of yeasts, influencing the saturation level and fluidity of yeast cell membranes. Further, the enhanced prostaglandin production in Candida non-albicans and drug resistant C. albicans as compared to C. albicans opens a new area of research to investigate the role of prostaglandin in antifungal resistance development and pathogenesis.
Conflicts of interestThe authors declare no conflicts of interest.
We thank the Director of CDRI and head of the Fermentation Technology Division, CSIR-CDRI, Lucknow, India for providing facilities. We also thank ICMR, New Delhi for giving me fellowship during this work CSIR-CDRI Communication No; 8541.