Geographical distribution of HIV variants is an important way to understand the circulation and spread of such viral strains.
ObjectivesTo evaluate the spatial distribution of HIV-1 variants in patients failing antiretroviral therapy, in Salvador, Brazil.
MethodsWe performed a cross-sectional evaluation of HIV resistance test reports of patients who underwent genotyping tests in a referral center in Salvador, Brazil, for the years 2008–2014. The laboratory database contains around 2500 resistance reports of patients failing antiretroviral therapy. Genotypic tests were performed by sequencing of HIV-1 POL region (TrueGene, Siemens). We assessed HIV-1 resistance mutations and subtype, as well as residential address, age, and gender of patients.
ResultsWe evaluated 1300 reports, 772 (59.4%) of them from male patients. As expected, subtype B predominated (79%) followed by subtypes F1 (6.7%) and BF (6.5%). The most frequent mutations in HIV-1 reverse transcriptase were 184V (79.1%), 41L (33.5%), 67N (30.4%), 103N (42.4%), and 108I (11.1%). Most frequent mutations in HIV-1 protease were 63P (52.4%), 36I (47.9%), 15 V (33.0%), 62 V (28.1%) and 13 V (25.8%). Some mutations (41L, 215Y, 210W) were significantly more frequent among men. We detected a significantly higher accumulation of 103N mutation in specific areas of Salvador. We identified a more restricted circulation pattern for subtype FB (more frequent in some regions), and F1 (almost absent in a specific region).
ConclusionOur results suggest that specific subtypes/resistance mutations present a distinct frequency rate in specific areas of Salvador, probably due to a restricted circulation pattern. This trend to clustering was observed in regions covered by AIDS referral centers, suggesting that pattern of care for such patients can interfere in virological outcomes.
The estimated number of people infected with HIV worldwide exceeds 34 million cases.1 Brazil has about 608,230 cases of infection (17.9/100,000 inhabitants).2 The Northeast region reported 12.9% (78,686) of cases, 19,290 (24.5%) of them in Bahia state.2 Salvador, state capital city, ranks third among Northeastern cities with the highest infection rate (28.9/100,000).2
One of the main obstacles to create a vaccine against HIV is the great genetic diversity presented by the virus, which varies according to different regions. These genetic mutations can be associated with different patterns of viral circulation and resistance to antiretrovirals.3–5 The epidemic in Bahia is characterized by a high genetic variability, with circulation of subtypes B, F, D and its recombinant forms.6,7 Social factors (population growth, urbanization, transportation, habits, and sexual behavior) contribute to the dispersion and to genetic diversity of the virus.8,9 Molecular epidemiology can be used to determine the history of viral evolution, the spatial spread, to identify the focus of viral dynamics, and how it takes place in a specific population.10–12
There are few studies in Latin America and Brazil about spatial dispersion of HIV-1 variants. It is extremely important to identify the geographical distribution of the virus in these countries, to understand its genetic diversity, and to evaluate potential factors driving the epidemic in specific areas. The objective of this study was to assess the spatial distribution of genetic mutations and viral subtypes of HIV-1 strains detected among patients failing antiretroviral therapy, in Salvador, Brazil.
MethodsStudy design, population, and settingsThis was a cross-sectional study. Genotypic test reports were retrieved from the database of the Laboratório de Pesquisa em Infectologia (Infectious Diseases Research Laboratory, LAPI), located at the University Hospital Professor Edgard Santos (HUPES), Federal University of Bahia, in Salvador, Brazil. Until February 2016, LAPI was the only public reference laboratory in Bahia to perform free of charge HIV resistance testing. To guarantee sample consistency we collected data for the years 2008–2014, since most of currently used antiretroviral drugs were already available in that period of time.
The HUPES is located in a central area of Salvador and serves predominantly patients from Salvador and surrounding cities. Data were collected from the LAPI genotyping database (which contains information on approximately 2500 patients), and included only those living in Salvador, with a residential address that could be identified by a valid postal code. A final sample of 1300 patients was included. We collected data on age, sex, residential address, and genotyping test reports. Genotyping was performed through a commercially available platform (TrueGene, Siemens, USA), according to manufacturer's instructions. HIV-1 drug-resistance mutations for each antiretroviral drug were assessed according to the IAS list.13
Statistical analysisThe collected information was stored in a database, and analyzed by using SPSS version 18 package. Frequency of HIV-1 subtypes and resistance mutations were expressed in percent, and adjusted for age, sex, and spatial location. The comparison between frequency of HIV-1 mutations/subtypes by sex/origin was performed by chi-square test. Mean age was compared using Student's t-test.
Georeference of HIV-1 variantsKernel density estimations and georeferencing was done using ArcGIS 10.3 (ESRI, 17 Redlands, CA, USA). Patients were grouped according to their informed postal code, in the different administrative regions of Salvador. Postal code was used to define patients geospatial localization.
ResultsPopulation and settingWe collected data from 1300 genotyping reports from February 2008 to December 2014. All individuals fulfilling the requirements to participate in the study had the genotyping test performed at LAPI. Among participants, 772 (59.4%) were men (mean age 42±9.4 years) and 528 (40.6%) were women (mean age 39±9.6 years, p=0.002, for comparison of mean age by sex).
We identified a total of 126 districts which were grouped according to the 16 administrative regions of the city of Salvador. We identified eight regions (listed in Table 1) as the origin of the majority (79%) of patients, while the remaining eight regions contributed to only 21% of the studied sample.
Most frequently detected resistance mutations associated with nucleoside reverse transcriptase inhibitors according to the administrative regions of Salvador, Brazil.
| Resistance mutation | Administrative regions | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | XI | XII | XVI | Other regions | |
| 41L | 38 (9%) | 37 (8%) | 49 (11%) | 58 (13%) | 46 (11%) | 46 (11%) | 38 (9%) | 41 (9%) | 83 (19%) |
| 67N | 41 (10%) | 33 (8%) | 35 (9%) | 53 (13%) | 38 (10%) | 49 (12%) | 41 (10%) | 41 (10%) | 72 (18%) |
| 70R | 29 (9%) | 26 (8%) | 26 (8%) | 39 (12%) | 32 (10%) | 46 (14%) | 32 (10%) | 31 (10%) | 57 (18%) |
| 184V | 95 (9%) | 103 (10%) | 84 (8%) | 124 (12%) | 87 (8%) | 135 (13%) | 86 (8%) | 110 (11%) | 193 (21% |
| 210W | 30 (10%) | 21 (7%) | 39 (13%) | 28 (10%) | 27 (9%) | 31 (11%) | 24 (8%) | 27 (9%) | 49 (18%) |
| 215Y | 29 (9%) | 26 (8%) | 38 (12%) | 25 (8%) | 29 (9%) | 40 (13%) | 19 (6%) | 45 (14%) | 60 (21%) |
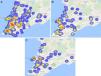
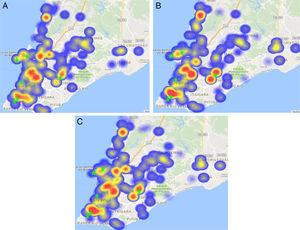
The main subtypes identified were B (78.5%), F1 (6.7%), and FB (6.5%), while the other subtypes were present in less than 3% of the studied patients. Subtype B was the most common variant and was present in all regions, with no significant variation in its geographical distribution. However, subtype FB predominated in regions II, III, IV and XVI, and was less frequent in regions V, XI and XII, as it can be seen in Fig. 1 (p=0.008).
On the other hand, subtype F1 was less frequent in region V, though this difference was only marginally non-significant as can be seen in Fig. 1 (p=0.064).
Resistance mutationsThe most frequent mutations associated with resistance to Nucleoside Reverse Transcriptase Inhibitors (NRTIs) were 184V (79.1%), 41L (33.5%), 67N (30.4%), 215Y (24.6%), 70R (24.5%), and 210W (22.3%). Other mutations had a frequency lower than 20%. The frequency of the thymidine-associated mutations (TAM) 41L and 210W was significantly higher among men than among women (34% vs. 29%, p=0.04, and 24% vs. 17%, p=0.002, respectively). Another TAM (215Y) showed a trend for a higher frequency among men than in women (25% vs. 21%, respectively, p=0.06).
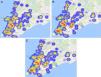
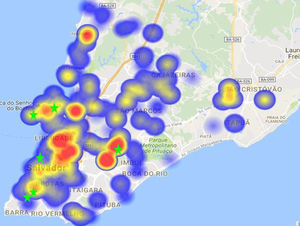
The distribution of the mutations associated to NRTI drugs can be seen in Table 1. No significant associations between NRTI-related mutations and specific regions were detected. However, there were several “hot spots” in the heat map for mutations 41L, 215Y, and 210W (Fig. 2)
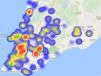
The most often detected mutations associated with resistance to non-nucleoside reverse transcriptase inhibitors (NNRTI) were 103N (42.4%), and 108I (11.1%). Other mutations of this group were detected in less than 10% of samples. There was no detected difference in mutations frequency according to gender. The distribution of NNRTI resistance mutations in the regions of Salvador is shown in Table 2. A significantly higher concentration of mutation 103N in region IV was observed, compared to other regions, as shown in Fig. 3 (p=0.002)
Most frequently detected resistance mutations associated with non-nucleoside reverse transcriptase inhibitors according to the administrative regions of Salvador, Brazil.
| Resistancemutation | Administrative regions | |||||||
|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | XII | XVI | Other regions | |
| 103N | 46 (8%) | 51 (9%) | 42 (8%) | 73 (13%) | 48 (9%) | 45 (8%) | 39 (7%) | 211(38% |
| 108I | 16 (11) | 20 (8%) | 16 (11%) | 19 (13%) | 7 (5%) | 8 (6%) | 13 (9%) | 58 (37%) |
The most frequent resistance mutations for Protease Inhibitors (PIs) were 63P (52.4%), 36I (47.9%), 15V (33.0%), 62V (28.1%), 77I (25.8%), 93L (25.3%), 71V (23.5%), 82A (22.2%), 46I (21.0%), and 10I (20.5%). Other detected mutations in this group had individual frequency lower than 20% (except for 13V polymorphism, 26% of frequency, p=0.001). The frequency of HIV-1 protease mutations was similar for male and female patients. The geographical distribution of PI resistance mutations is shown in Table 3.
Most frequently detected resistance mutations associated with protease inhibitors according to the administrative regions of Salvador, Brazil.
| Resistance mutation | Administrative regions | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | XI | XII | XVII | Other regions | |
| 15V | 30 (7%) | 43 (10%) | 41 (10%) | 58 (14%) | 26 (6%) | 55 (13%) | 37 (9%) | 43 (10%) | 88 (21%) |
| 36I | 52 (8%) | 61 (10%) | 44 (7%) | 83 (13%) | 53 (9%) | 73 (12%) | 52 (8%) | 68 (11%) | 137 (22%) |
| 62V | 32 (8%) | 33 (9%) | 30 (8%) | 53 (14%) | 45 (12%) | 42 (11%) | 28 (7%) | 33 (9%) | 83 (22%) |
| 63P | 49 (7%) | 59 (9%) | 59 (9%) | 76 (11%) | 67 (10%) | 77 (11%) | 66 (10%) | 61 (9%) | 180 (26%) |
As observed in Fig. 2, there was a specific pattern for geographical location of distinct mutations, but all of them were concentrated in six districts.
Again, there was an overlapping area comprising the regions II, III, IV, and XVI that presented significantly higher frequencies of subtypes F1 and FB. In addition, a neighboring area (region V) showed the lower frequency of subtype F1. Of note, we observed that the “hot spots” for most resistance mutations were apparent in the neighborhood of three main referral centers for AIDS care in Salvador (pointed by green stars in Figs. 1–3)
DiscussionIn the present work resistance mutations were significantly more frequent in older men failing ART. We detected a predominance of HIV-1 subtype B, followed by subtypes FB and F1, among patients failing ARV therapy in Salvador, Brazil. Other subtypes were detected at a lower frequency in the studied patients. Subtype B was similarly distributed across the regions of the city, but FB subtype presented two distinct profiles, with a higher concentration of cases in regions II, III, IV, and XVI, in comparison to regions V, XI, XII. Subtype F1 had a similar distribution across regions, except in region V, where it was less frequently detected than other subtypes, but the difference was not statistically significant.
We observed a trend for a higher frequency of NRTIs-associated resistance mutations 41L, 215Y, and 210W in regions III, IV and XI of Salvador, although without reaching statistical significance. However, mutations 41L and 210W were significantly associated with male sex, while a third TAM (215Y) was also more frequent among men, although this association was only marginally significant. The most frequently detected NNRTIs associated resistance mutation (103N) also differed in terms of geographical location, with a significant concentration in a specific region. The frequency of the main subtypes observed in this work is in agreement with data found by previous studies, and confirms that the most prevalent subtype in Salvador is subtype B. However, a higher frequency of FB and F1 subtypes was observed, in comparison with previous studies.5,6 Our patients were predominantly males, in contrast to the findings of Davanos,14 in Greece, but in accordance to the gender distribution of AIDS patients in Brazil.2 In addition, males were more likely to present NRTI-related resistance mutations, while no difference was observed for PI/NNRTI-related mutations. On the other hand, NNRTI specific mutations were more frequently detected in a few administrative regions. These findings indicate that social and behavioral factors may influence the onset of specific mutations, as already suggested by Tomazelli.15
The combined results for drug-resistance mutations and HIV-1 subtypes, that significantly differ according to spatial localization, shows a common location for all these clusters, the Liberdade district (region IV), which is present in all different clusters observed. This specific district is one of the most populous in Salvador, and has a low income, predominantly black population. This is the only common site for all different clusters identified in this study which suggest it is the likely epicenter for spreading of these distinct HIV-1 variants. Also, the coincident geographical locations of the detected “hot-spots” and referral centers for AIDS care (pointed by green stars at Fig. 3) offer a potential explanation for these findings, as they can reflect the prescription pattern of each institution.
Based on this findings, one could postulate that sociodemographic characteristics can influence the risk of HIV infection and, probably, of failing therapy. In the present work, we detected a pattern of viral variants with a predominant circulation in the poorer areas of Salvador, reinforcing some previous findings.16 In addition, the detected difference in the frequency of some mutations according to sex also indicates the importance of social factors on HIV variability and dissemination. Questions like differential adherence and tolerability for specific drugs may explain the differences between males and females, with regard to frequency of TAMs.17–19
A similar pattern was detected in South Africa where Tanser et al. observed a tendency for grouping HIV-1 subtypes in more populated districts.20 Other studies in Sub-Saharan Africa showed a pattern of the spread of HIV subtypes in areas of greater spatial mobility, linked by easy-access connecting ways.21,22 In a different scale we could observe the same pattern among regions of Salvador, with a trend for clustering of some mutations subtypes in interconnected areas, with a similar socio-economic profile.
Similar findings were also detected in Uganda and Rio de Janeiro, where genetic variants of HIV-1 had a distinct pattern of distribution over time, in neighboring regions, suggesting that even transmissibility and pathogenesis could be affected by these aspects.23,24 In common, all studied regions have the same low income profile and low development level. Lower socio-economic levels are usually associated with decreased adherence to therapy, and higher risk of virological failure.25–27 Taken together, these findings suggest that living conditions can impact treatment outcomes.
Brazil was one of the first countries to provide free of charge access to care and treatment for people infected by HIV. Recently, the Brazilian AIDS Program started to treat all viremic patients, regardless of CD4 count or clinical status. In this context, understanding the viral dynamics in AIDS epidemic is a crucial step, to design effective treatment and prevention strategies.28 As proposed by Mann, the HIV epidemic seems to be the result of several outbreaks occurring in different segments of society and there are different speed and factors influencing HIV-1 dissemination patterns.29
Salvador is a 3-million inhabitants city, with a low average income. Most patients failing ARV therapy in our study came from the poorer areas of the city, and the trend for clustering some genetic variants of HIV follows the same pattern. Also, these mutations were more prevalent in the population living in the vicinity of specific referral centers for AIDS care, which can add the quality of received medical care as an important factor associated with resistance to ARV drugs.
Our study presents some clear limitations: due to the retrospective design, many sociodemographic variables were not available, limiting the reach of our results and conclusions. In addition, detailed information on previous use of antiretroviral drugs would provide some important background information, but we did not have access to such data. Finally, to confirm the relatedness of viral strains and its restricted spatial circulation we should have adequate phylogenetic analysis, not present in this work. However, the description of a geographical pattern of HIV variants circulation is an important tool to understand the dynamic of viral dissemination in a context of patients failing therapy, and provide useful information that can be translated in different strategies to prevent HIV resistance transmission.
Although the detected drug-resistance mutations basically reflect the previous use/failure of specific ARV regimens, its transmission can be affected by social and geographical characteristics of patients. The circulation of certain HIV-1 variants in restricted areas can require specific preventive measures to face the increasing number of resistant strains that can be transmitted to other subjects, and may help designing optimal treatment strategies for both, naïve and experienced patients. Identifying the driving forces that impact the onset of drug-resistance in patients failing therapy may be of help in developing strategies to improve medical care, and preventive measures that can reduce viral resistance among AIDS patients on ART.
Conflicts of interestThe authors declare no conflicts of interest.