Gram-negative bacilli (GNB), notably Acinetobacter spp., Pseudomonas spp., and Klebsiella spp., are becoming increasingly resistant to carbapenems and are associated with high health care costs and mortality, becoming a global concern.
ObjectiveTo determine the prevalence rates of carbapenem resistance among Acinetobacter spp., Pseudomonas spp., and Klebsiella spp. in the main sites of nosocomial infection at a tertiary care hospital in southern Brazil and the consequent therapeutic implications.
MethodsCultures processed at the institution’s laboratory in 2017 were analyzed, and those positive for Acinetobacter spp., Pseudomonas spp., and Klebsiella spp. were identified. Antibiograms were evaluated for meropenem sensitivity following the Clinical Laboratory Standards Institute guidelines.
ResultsAcinetobacter spp. had the lowest prevalence among the three GNB, and resistance of this pathogen to meropenem at different sites of infection ranged from 36% (blood) to 82% (respiratory tract). Pseudomonas spp. was highly prevalent at the respiratory tract (31%) and had a high resistance rate to meropenem in rectal swab samples (71%), but a relatively low frequency at infection sites (skin/soft tissue, 13%; blood, 25%). Klebsiella spp. was identified in 7.5% of the blood cultures and 15% of the urine cultures and was the chief colonizer among all pathogens, representing 54% of all rectal swab samples, of which 53% were meropenem resistant. At sites of infection, rates of Klebsiella spp. resistant to meropenem ranged from 19% (skin) to 55% (vascular catheter).
ConclusionsThe prevalence of carbapenem-resistant GNB at our hospital was relatively low compared to national and international data; thus, meropenem remains a good therapeutic option against these bacteria. Other antibiotics effective against GNB, such as ceftazidime, cefepime, and piperacillin-tazobactam, can be used in most cases, while meropenem should be reserved for patients with sepsis. Strict contact precaution measures are still needed, given the high resistance rate observed at the colonizing site.
Antimicrobial resistance is a natural process that is being exacerbated mainly by the indiscriminate use of antimicrobials1 in the medical field (e.g., unnecessary prescriptions, incorrect administration, and over-the-counter sales2) and activities related to agriculture and livestock (use of antibiotics for animal growth).3
Infections by antimicrobial-resistant organisms are associated with increased mortality. These pathogens also curb empirical treatment of infections, raise costs associated with health care, and limit therapeutic options to agents with lower efficacy and more side effects.4,5 According to the World Health Organization (WHO), this scenario threatens the global public health with the prospect of a post-antibiotic era when antibiotics will lose efficacy to treat infections; this could affect all medical areas and increase global morbidity and mortality rates.6
Among all pathogens involved in multidrug-resistant infections, Gram-negative bacilli (GNB) – mainly Acinetobacter spp., Pseudomonas spp., and Klebsiella spp.7,8 – are the most threatening, since no new class of antibiotics has been developed against these bacteria.9 Additionally, the rates of resistance to carbapenems (the current drug of choice for multidrug-resistant infections) have increased mainly due to KPC, NDM, and OXA-48 carbapenemases, making the management of infections by these bacteria a concerning public health problem worldwide.7 Beta-lactam agents like carbapenems are especially important in the management of patients in transplant and intensive care units (ICUs), and among individuals on chemotherapy, since multidrug-resistant (MDR) GNB are a frequent cause of sepsis in these environments.10
Cases of resistance to carbapenems are managed by long-established drugs such as aminoglycosides, tigecycline, colistin, and polymyxin. In addition to the potential side effects of these medications, including hearing loss and kidney failure, resistance rates to these drugs have increased along with the emergence of pandrug-resistant (PDR) organisms.10
According to the WHO Global Action Plan, one of the measures to prevent the advance of antimicrobial resistance is the generation and sharing of epidemiological information reporting the particularities of each region.6 Considering the importance of this measure and the fact that Brazil is part of BRICS (which forecasts an increase in the use of antimicrobials),11 this study was developed to evaluate the prevalence and profile of susceptibility to carbapenems among the GNB Acinetobacter spp., Pseudomonas spp., and Klebsiella spp. and the prevalence of carbapenem resistance among these GNB at a tertiary hospital in Curitiba (the eighth most populous city in Brazil, according to the Brazilian Institute of Geography and Statistics),12 along with addressing their consequent therapeutic implications.
Materials and methodsThis was a retrospective, cross-sectional study in patients who had cultures processed during 2017 at the Clinical Analysis Laboratory Unit (ULAC) of the Clinics Hospital Complex of Federal University of Paraná (CHC-UFPR), in Curitiba, southern Brazil. The CHC-UFPR is a large tertiary care hospital with 500 hospital beds (85 of which are distributed across three ICUs: adult, pediatric, and neonatal), obstetric and surgical centers, and outpatient clinics of various specialties. The hospital is a referral center for some specialties (bone marrow transplantation, hematologic diseases, and cystic fibrosis) and offers care for patients across all ages and races, of both sexes, and with diseases with and without comorbidities.
The cultures were routinely processed in the laboratory following standard microbiology methods. Identification of the bacteria and antimicrobial susceptibility tests were performed using the automated system Vitek 2 (bioMérieux S.A., Marcy-l’Étoile, France) and interpreted according to Clinical Laboratory Standards Institute (CLSI) guidelines. Meropenem was chosen to represent the carbapenem class since it is the most frequently used antibiotic of this class.
The study included 1142 positive cultures for Pseudomonas spp., Acinetobacter spp., and Klebsiella spp. obtained from patients across all ages. Cultures from samples obtained from outpatients were excluded. A total of 1121 were included in the prevalence analysis, while 21 were excluded from this analysis since the samples were collected from sites and media other than those analyzed in the present study (blood, urine, respiratory tract, abdomen, rectum, skin/soft tissues, and vascular catheter). Moreover, for the sensitivity analysis 30 samples were excluded due to absence of results of meropenem susceptibility tests, resulting in 1112 samples for this specific analysis.
We estimated the prevalence and resistance rates of each bacteria and calculated the prevalence rates of carbapenem resistance at each site of infection and colonization.
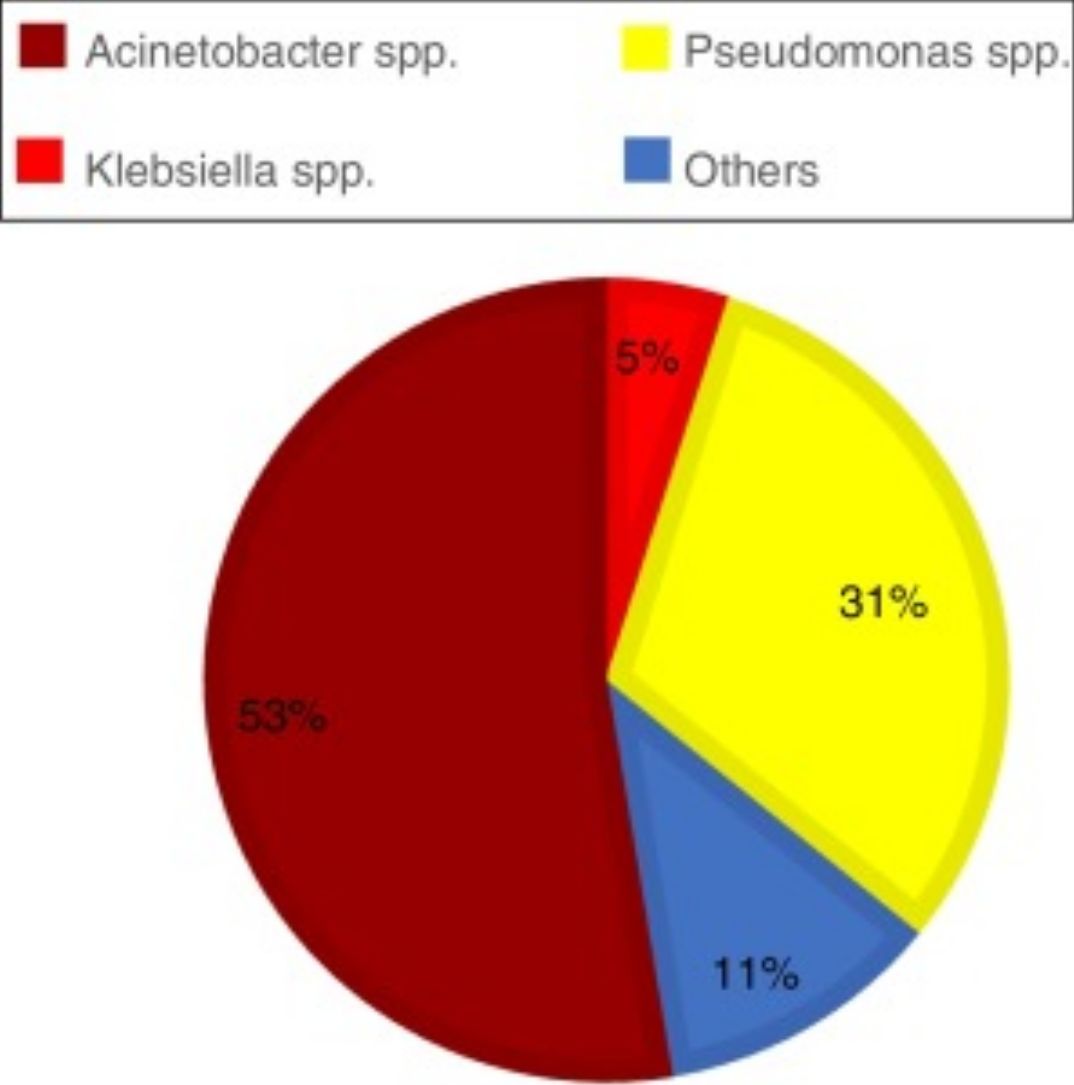
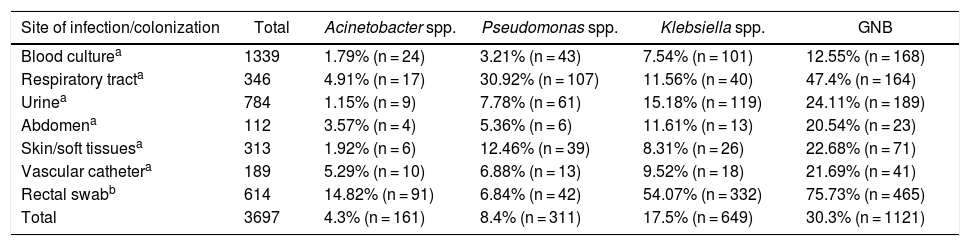
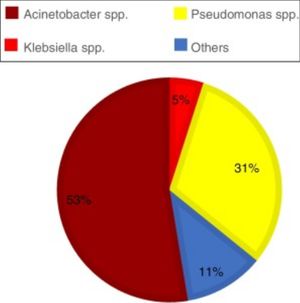
ResultsAcross all sites and media analyzed, blood cultures had the lowest prevalence of the three GNB combined (12.55%). The site with the highest prevalence of all three GNB was the respiratory tract, where these bacteria represented almost half of all infections; of note, Pseudomonas spp. was identified in approximately one-third of all cultures from the respiratory tract (Graph 1). The three GNB also represented nearly a quarter of urine cultures and three-quarters of rectal swab cultures. The prevalence of these bacteria at other sites was around 20% (Table 1).
Prevalence of Gram-negative bacilli (GNB) by site of infection/colonization.
| Site of infection/colonization | Total | Acinetobacter spp. | Pseudomonas spp. | Klebsiella spp. | GNB |
|---|---|---|---|---|---|
| Blood culturea | 1339 | 1.79% (n = 24) | 3.21% (n = 43) | 7.54% (n = 101) | 12.55% (n = 168) |
| Respiratory tracta | 346 | 4.91% (n = 17) | 30.92% (n = 107) | 11.56% (n = 40) | 47.4% (n = 164) |
| Urinea | 784 | 1.15% (n = 9) | 7.78% (n = 61) | 15.18% (n = 119) | 24.11% (n = 189) |
| Abdomena | 112 | 3.57% (n = 4) | 5.36% (n = 6) | 11.61% (n = 13) | 20.54% (n = 23) |
| Skin/soft tissuesa | 313 | 1.92% (n = 6) | 12.46% (n = 39) | 8.31% (n = 26) | 22.68% (n = 71) |
| Vascular cathetera | 189 | 5.29% (n = 10) | 6.88% (n = 13) | 9.52% (n = 18) | 21.69% (n = 41) |
| Rectal swabb | 614 | 14.82% (n = 91) | 6.84% (n = 42) | 54.07% (n = 332) | 75.73% (n = 465) |
| Total | 3697 | 4.3% (n = 161) | 8.4% (n = 311) | 17.5% (n = 649) | 30.3% (n = 1121) |
Abbreviation: GNB: Gram-negative bacilli.
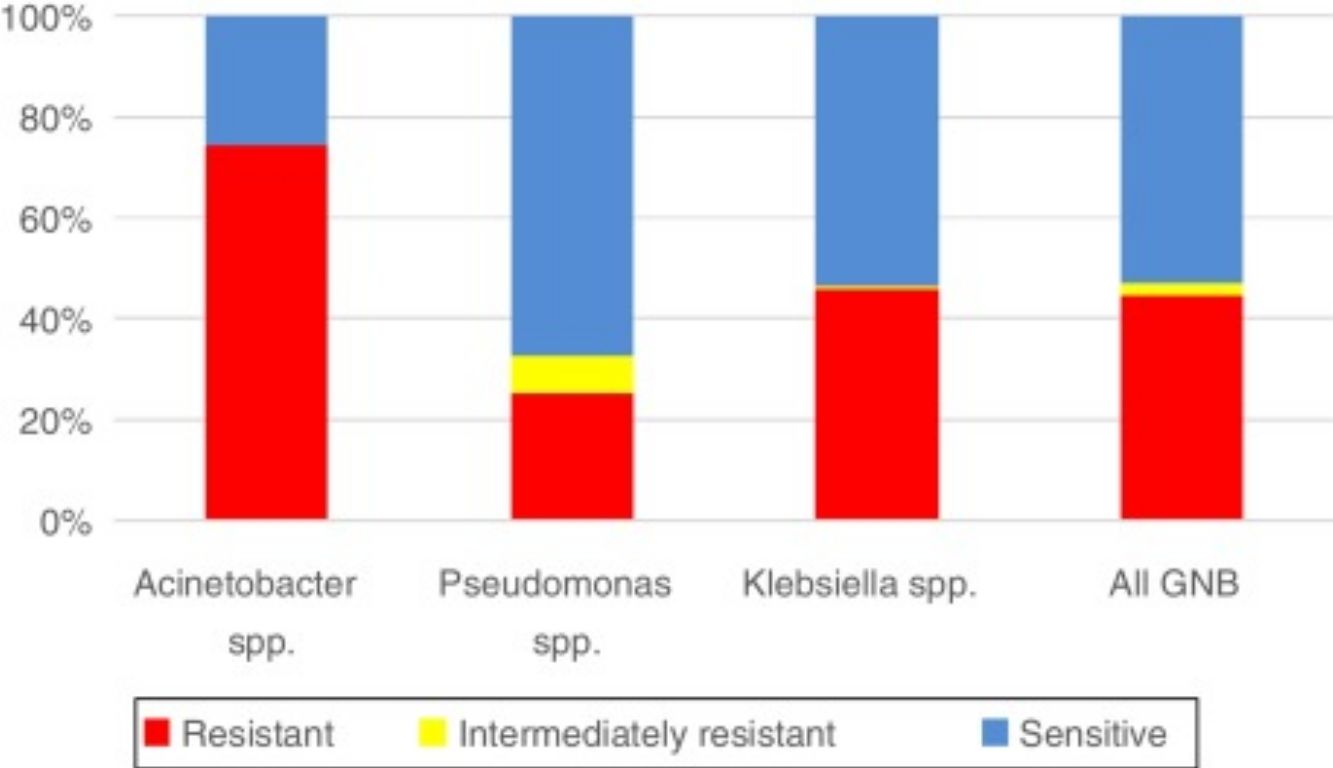
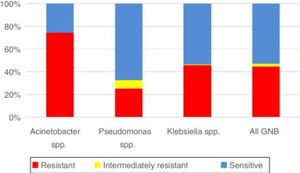
With respect to resistance to carbapenem among the three pathogens, the rate was highest for Acinetobacter spp. (74.55%, n = 123), followed by Klebsiella spp. (45.79%, n = 299), and Pseudomonas spp. (25.17%, n = 74). According to overall susceptibility to meropenem among all three GNB, 53.06% (n = 590) were sensitive, 2.34% (n = 26) were intermediately resistant, and 44.6% (n = 496) were resistant to this antibiotic (Graph 2).
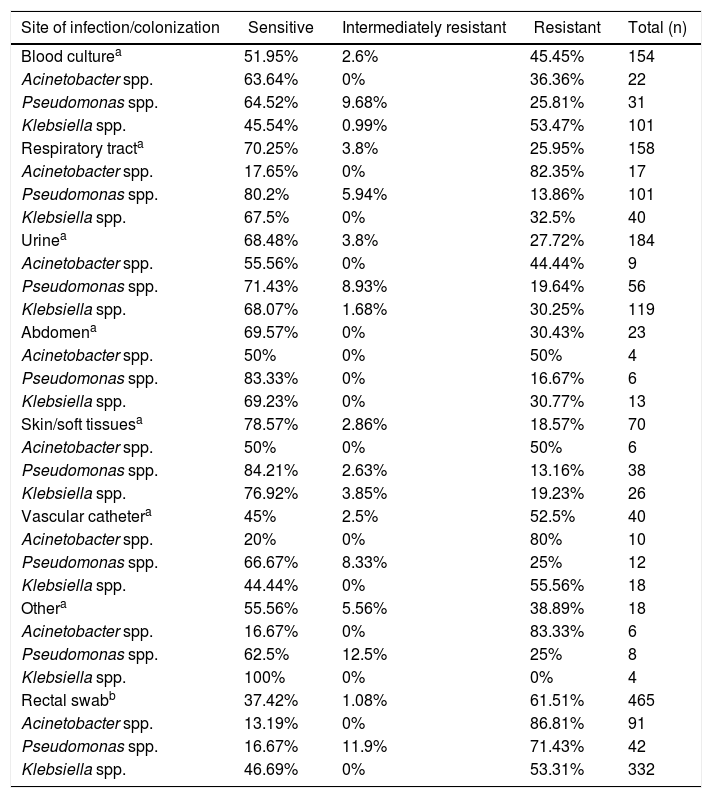
Table 2 shows the rates of resistance to meropenem across all three bacteria analyzed according to infection and colonization sites/media. The highest resistance rate (61.51%) was observed in the colonization site (rectum) and mostly for Klebsiella spp. Resistance of Acinetobacter spp. to meropenem at various infection sites/media ranged from 36.36% (blood) to 82.35% (respiratory tract); resistance at the colonization site (rectum) was 86.81%. Resistance of Pseudomonas spp. to meropenem was high in the colonization site (71.43%), but relatively low in other sites/media, ranging from 13.16% in skin/soft tissues to 25.81% in blood. The rate of meropenem resistance among Klebsiella spp. in the colonization site was 53.31%, while at infection sites/media, resistance to this agent ranged from 19.23% (skin/soft tissues) to 55.56% (vascular catheter).
Susceptibility profile of Gram-negative bacilli (GNB) by site of infection/colonization.
| Site of infection/colonization | Sensitive | Intermediately resistant | Resistant | Total (n) |
|---|---|---|---|---|
| Blood culturea | 51.95% | 2.6% | 45.45% | 154 |
| Acinetobacter spp. | 63.64% | 0% | 36.36% | 22 |
| Pseudomonas spp. | 64.52% | 9.68% | 25.81% | 31 |
| Klebsiella spp. | 45.54% | 0.99% | 53.47% | 101 |
| Respiratory tracta | 70.25% | 3.8% | 25.95% | 158 |
| Acinetobacter spp. | 17.65% | 0% | 82.35% | 17 |
| Pseudomonas spp. | 80.2% | 5.94% | 13.86% | 101 |
| Klebsiella spp. | 67.5% | 0% | 32.5% | 40 |
| Urinea | 68.48% | 3.8% | 27.72% | 184 |
| Acinetobacter spp. | 55.56% | 0% | 44.44% | 9 |
| Pseudomonas spp. | 71.43% | 8.93% | 19.64% | 56 |
| Klebsiella spp. | 68.07% | 1.68% | 30.25% | 119 |
| Abdomena | 69.57% | 0% | 30.43% | 23 |
| Acinetobacter spp. | 50% | 0% | 50% | 4 |
| Pseudomonas spp. | 83.33% | 0% | 16.67% | 6 |
| Klebsiella spp. | 69.23% | 0% | 30.77% | 13 |
| Skin/soft tissuesa | 78.57% | 2.86% | 18.57% | 70 |
| Acinetobacter spp. | 50% | 0% | 50% | 6 |
| Pseudomonas spp. | 84.21% | 2.63% | 13.16% | 38 |
| Klebsiella spp. | 76.92% | 3.85% | 19.23% | 26 |
| Vascular cathetera | 45% | 2.5% | 52.5% | 40 |
| Acinetobacter spp. | 20% | 0% | 80% | 10 |
| Pseudomonas spp. | 66.67% | 8.33% | 25% | 12 |
| Klebsiella spp. | 44.44% | 0% | 55.56% | 18 |
| Othera | 55.56% | 5.56% | 38.89% | 18 |
| Acinetobacter spp. | 16.67% | 0% | 83.33% | 6 |
| Pseudomonas spp. | 62.5% | 12.5% | 25% | 8 |
| Klebsiella spp. | 100% | 0% | 0% | 4 |
| Rectal swabb | 37.42% | 1.08% | 61.51% | 465 |
| Acinetobacter spp. | 13.19% | 0% | 86.81% | 91 |
| Pseudomonas spp. | 16.67% | 11.9% | 71.43% | 42 |
| Klebsiella spp. | 46.69% | 0% | 53.31% | 332 |
Abbreviation: GNB: Gram-negative bacilli.
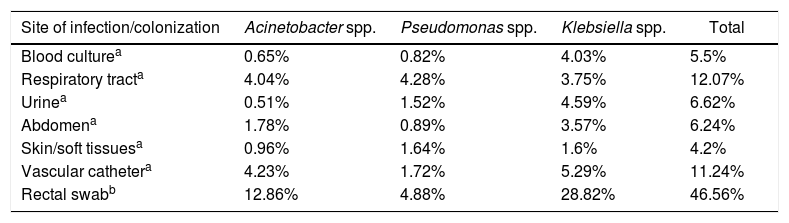
Regarding the three GNB analyzed, the prevalence of carbapenem (meropenem) resistance ranged from 4.2% at skin/soft tissues to 12.07% at the respiratory tract; at the colonization site (rectum), this rate was 46.56%. At sites of infection, carbapenem-resistant Acinetobacter spp. and Klebsiella spp. were more prevalent in vascular catheter cultures, while carbapenem-resistant Pseudomonas spp. was more prevalent in cultures of samples obtained from the respiratory tract (Table 3).
Prevalence of carbapenem-resistant Gram-negative bacilli (CR-GNB).
| Site of infection/colonization | Acinetobacter spp. | Pseudomonas spp. | Klebsiella spp. | Total |
|---|---|---|---|---|
| Blood culturea | 0.65% | 0.82% | 4.03% | 5.5% |
| Respiratory tracta | 4.04% | 4.28% | 3.75% | 12.07% |
| Urinea | 0.51% | 1.52% | 4.59% | 6.62% |
| Abdomena | 1.78% | 0.89% | 3.57% | 6.24% |
| Skin/soft tissuesa | 0.96% | 1.64% | 1.6% | 4.2% |
| Vascular cathetera | 4.23% | 1.72% | 5.29% | 11.24% |
| Rectal swabb | 12.86% | 4.88% | 28.82% | 46.56% |
Abbreviation: CR-GNB: carbapenem-resistant Gram-negative bacilli.
Acinetobacter spp., Pseudomonas spp., and Klebsiella spp. are frequently isolated in samples collected from patients, reflecting their importance as human pathogens. Rates of carbapenem resistance in the present study varied according to species and infection site, and although the rates found were lower than those reported in other similar institutions, they are still concerning due to the scarcity of therapeutic options.
The bacteria with the highest rate of resistance to carbapenem were Acinetobacter spp. (74.55%) (Graph 2). Acinetobacter spp. (mainly A. baumannii) is usually resistant to multiple drugs, and rates of carbapenem resistance among these pathogens have been described at 98.1% in China (2013),13 91% in Greece (2014),14 95% in India (2015–2016),15 75.5% in Latin American countries (2011),16 and 76.8–100% in Brazil.17,18 The lower rates of Acinetobacter spp. resistance to carbapenem found in our study may indicate that infections by these pathogens are well managed at our institution.
The bacteria with the lowest but nevertheless concerning resistance rate in our study were Pseudomonas spp. (25.17%) (Graph 2). This frequency was lower than the rates reported in Latin America in general (38.4%), Guatemala (75.8%), Peru (62.5%), and Ecuador (55.6%) in 201116 and in ICUs in India between 2015 and 2016 (56%),15 and was more aligned with rates found in Spain in 2013 (24.52%)19 and other countries such as Poland, Lithuania, Slovakia, Hungary, Croatia, Romania, Bulgaria, and Greece, where the frequency of carbapenem resistance ranges from 25% to 50%.20
The rate of Klebsiella spp. Resistance to meropenem was 45.79% (Graph 2), which is similar to the rate described in Rio Grande do Sul in 2016 (52.6%)21 but higher than that reported in São Paulo in 2015 (35.5%).22 Additionally, this rate is lower than the one documented in Greece in 2014 (62.3%).23 In bacteremias (blood cultures), Klebsiella spp. had a resistance rate of 53.47%, which is higher than the rates reported in Italy (15% in 2010 and 32.3% in 2014) and Greece (27.8% in 2005), but lower than the rate described in Greece in 2014 (62.3%).20
Cultures of vascular catheter and blood had the highest rates of carbapenem resistance considering all three GNB combined (52.5% and 45.45%, respectively) (Table 2). Vascular catheters and blood are the most concerning infection sites for carbapenem-resistant GNB. The sites and media with GNB with highest sensitivity rates to meropenem were the skin/soft tissues (78.57%), respiratory tract (70.25%), abdomen (69.57%), and urinary tract (68.48%) (Table 2), indicating that meropenem is more effective at these sites.
All sites associated with hospital-related infections had a relatively small prevalence of meropenem-resistant GNB. The following are recommendations of antibiotics with activity against GNB, keeping in mind that bacteremias and skin/soft tissue and vascular catheter infections should also cover Gram-positive cocci, with methicillin-resistant Staphylococcus aureus (MRSA) generally being the most prevalent MDR bacteria at these sites.
The three GNB together accounted for 12.55% of all bacteria identified in blood cultures, and the rate of carbapenem resistance among these GNB was 5.4%. The most prevalent of all three GNB was Klebsiella spp.; these pathogen represented 7.54% of all bacteria causing bacteremia (Table 3), of which 53.47% were resistant to meropenem (Table 2). Thus, only 4.03% of the bacteremias at our institution in 2017 were caused by meropenem-resistant Klebsiella spp. (Table 3). Given this low prevalence of carbapenem resistance, antibiotics with a narrower antibacterial spectrum than meropenem (such as cefepime and piperacillin-tazobactam) are suitable for patients without sepsis and with GNB positive blood culture while bacteria identification and antibiogram are pending.20 In patients with sepsis, meropenem is the right therapeutic choice, while antibiotics such as polymyxin B, aminoglycosides, ceftolozane-tazobactam and ceftazidime-avibactam should be reserved for meropenem-resistant GNB20,24–26 and only for targeted treatment, i.e., when sensitivity tests are available and demonstrate resistance to meropenem.
Cultures of samples obtained from the respiratory tract had a high prevalence of Pseudomonas spp. (31%) (Graph 1), with only 13.86% resistant to meropenem (Table 2). Klebsiella spp. were the second most prevalent GNB in the respiratory tract (11.56%) (Table 1). When combined, the three GNB showing resistance to meropenem accounted for 12.07% of the cultures of samples obtained from the respiratory tract (Table 3). This suggests that meropenem can be used for empirical treatment, while polymyxin B, aminoglycosides, and ceftazidime-avibactam should be reserved for treatment of carbapenem-resistant GNB.
The three GNB surveyed accounted for 24.11% of all positive urine cultures, and the prevalence of carbapenem resistance among these GNB cultured from the urinary tract was 6.62% (Table 3). Klebsiella spp. were isolated from 15.18% of the urine cultures, which is roughly twice the prevalence of Pseudomonas spp. in these cultures, the second of all three GNB surveyed (Table 1). Aminoglycosides, ceftazidime, cefepime, or piperacillin-tazobactam20 are all suitable antibiotics in most cases of urinary tract infection caused by these three GNB.
Among intra-abdominal infections, Klebsiella spp. were the most frequent pathogens identified (11.1% of the total) (Table 1) and were resistant to meropenem in 30.77% of the cases (Table 2). The prevalence of carbapenem-resistant GNB in intra-abdominal samples was only 6.24% (Table 3). Among alternatives for intra-abdominal infections, cefepime combined with metronidazole or piperacillin-tazobactam20 are good options, while meropenem should be reserved for patients with sepsis.
The three GNB analyzed represented 22.68% of the total samples obtained from skin/soft tissues (Table 1); among these, only 4.2% were resistant to carbapenem (Table 3). Cefepime or piperacillin-tazobactam20 are more suitable antibiotics for empirical treatment. Polymyxin B, aminoglycosides, and ceftazidime-avibactam are probably the best choices for targeted treatment of carbapenem-resistant GNB.
Gram-positive cocci predominated in vascular catheter infections. In all, 11.24% of all positive vascular catheter cultures were caused by a combination of the three GNB showing carbapenem resistance (Table 3). This finding reinforces the use of antibiotics reserved for carbapenem resistance only when resistance is confirmed by susceptibility testing.
Among positive cultures of rectal swab – which represent colonization rather than infection – the combined rates of carbapenem resistance among the three GNB analyzed was 46.56% (Table 3). Among these, Klebsiella spp. was isolated from 54.07% of the cases (Table 1), of which just over half (53.31%) were resistant to meropenem (Table 2). This high prevalence of rectal swab samples from patients colonized by carbapenem-resistant GNB shows the importance of infection control measures at the hospital level to prevent the spread of these bacteria as a potential cause of health care-related infections.
ConclusionsConsidering the complexity of the patients seen at the CHC-UFPR, the rates of carbapenem-resistant GNB were relatively low when compared to national and international rates. This probably indicates that the infection control measures taken at the hospital are currently effective, but should not forego the adoption of more strict contact precautions measures (mainly due to the high prevalence of patients colonized by carbapenem-resistant GNB) and the use of alternative antibiotic therapies to spare carbapenems whenever possible. Meropenem remains a good empirical therapeutic option to treat patients with severe hospital-acquired infection (sepsis) at CHC-UFPR. The correct use of antibiotics is essential to avoid the consequences of inadequate treatment, such as increased mortality and hospital costs, along with the dissemination of MDR bacteria.
Conflicts of interestThe authors declare no conflicts of interest.