To collect data about non-controlled prescribing use of daptomycin and its impact among Brazilian patients with serious Gram positive bacterial infection, as well as the efficacy and safety outcomes.
Materials and methodsThis is a multi-center, retrospective, non-interventional registry (August 01, 2009 to June 30, 2011) to collect data on 120 patients (44 patients in the first year and 76 patients in the second year) who had received at least one dose of commercial daptomycin in Brazil for the treatment of serious Gram-positive bacterial infection.
ResultsRight-sided endocarditis (15.8%), complicated skin and soft tissue infections (cSSTI)-wound (15.0%) and bacteremia-catheter-related (14.2%) were the most frequent primary infections; lung (21.7%) was the most common site for infection. Daptomycin was used empirically in 76 (63.3%) patients, and methicillin-resistant Staphylococcus aureus (MRSA) was the most common suspected pathogen (86.1%). 82.5% of the cultures were obtained prior to or shortly after initiation of daptomycin therapy. Staphylococcus spp. – coagulase negative, MRSA, and methicillin-susceptible S. aureus were the most frequently identified pathogens (23.8%, 23.8% and 12.5%, respectively). The most common daptomycin dose administered for bacteremia and cSSTI was 6mg/kg (30.6%) and 4mg/kg (51.7%), respectively. The median duration of inpatient daptomycin therapy was 14 days. Most patients (57.1%) did not receive daptomycin while in intensive care unit. Carbapenem (22.5%) was the most commonly used antibiotic concomitantly. The patients showed clinical improvement after two days (median) following the start of daptomycin therapy. The clinical success rate was 80.8% and the overall rate of treatment failure was 10.8%. The main reasons for daptomycin discontinuation were successful end of therapy (75.8%), switched therapy (11.7%), and treatment failure (4.2%). Daptomycin demonstrated a favorable safety and tolerability profile regardless of treatment duration.
ConclusionsDaptomycin had a relevant role in the treatment of Gram-positive infections in the clinical practice setting in Brazil.
Gram-positive bacteria are a major cause of complicated skin and soft tissue infections (cSSTI) as well as etiologic agent of bacteremia with or without infective endocarditis, with Staphylococcus aureus (including methicillin-resistant [oxacillin-resistant] S. aureus), Streptococcus species, and Enterococcus species being the most common pathogens. cSSTI are considered those in deep soft tissue, or requiring surgical intervention (infected ulcers, burns, major abscesses), or associated with comorbidities (diabetes mellitus, obesity, immune deficiency, underlying venous or arterial insufficiency).1 Methicillin-resistant S. aureus (MRSA) and methicillin-resistant coagulase-negative Staphylococcus (MR-CoNS) are well-recognized causes of both community-acquired and healthcare-associated infections.2 MRSA infections have risen in the last few years. In Europe, the reported rate of MRSA is as low as 5% in Denmark, Finland, Netherlands, and Sweden and as high as above 40% in Greece, Ireland, Italy, Malta and the UK.3 In USA,4 MRSA caused 53% of all S. aureus healthcare-associated infections reported to NHSN in 2006–2007, ranging from 49.2% of S. aureus SSIs to 65.2% of S. aureus catheter-associated urinary tract infections (CAUTI). In Brazil, the overall MRSA rate was 31%.5 Coagulase-negative staphylococci (CoNS) are important etiologic agents of bacteremia among immunocompromised patients, mainly when they are using catheters. They are increasingly involved in infective endocarditis,6 greatly associated with the use of intravenous catheters.
Although vancomycin has been considered the first-line drug of choice for treating MRSA and MRCoNS, evidence of clinical failures are increasing, suggesting that vancomycin is losing its clinical and microbiological potency, resulting in increased use of novel antibiotics such as daptomycin.7 These poor vancomycin results have been more evident when staphylococcal infections are associated to bacteremia.
The objective of the daptomycin registry in Brazil was to collect data about non-controlled prescribing use of daptomycin and its the impact in Brazilian patients with serious Gram positive bacterial infection, as well as the efficacy and safety outcomes.
Materials and methodsEU-CORE was a multi-center, retrospective, non-interventional registry over a five-year (2007–2012) period to annually collect outcome data on patients who have received at least one commercial dose of daptomycin for the treatment of serious Gram-positive bacterial infection. Collected data concerned patient population, infections, pathogens, adverse events (AEs) and clinical outcomes. Brazil participated during three years, from 2009 to 2012 and this is the report of the first two years of participation, from August 01, 2009 to July 31, 2010 and from August 01, 2010 to July 31, 2011. During these two years, Brazil included 120 patients in the study, being 44 patients in the first year and 76 patients in the second year.
The sample size of this patient registry is not based on statistical considerations. The primary study objectives were to characterize and describe the population of patients receiving daptomycin and the infections and pathogens treated with daptomycin; to evaluate the clinical outcomes of daptomycin therapy; to characterize, describe and evaluate AEs in patients receiving daptomycin. Data for efficacy assessment were collected for clinical outcome assessment, duration of treatment, time to clinical improvement, and safety issues (AE/serious AE [SAE]). The outcome of clinical improvement was considered in the case of partial resolution of clinical signs and symptoms and/or when there was a need for additional antibiotic therapy at the end of daptomycin therapy; failure was defined as inadequate response to daptomycin therapy or development of resistance, worsening or new/recurrent signs and symptoms, or the need to change antibiotic therapy, or a positive culture reported at the end of therapy.
The inclusion criteria for a patient record to be eligible for retrospective data collection and inclusion in the registry database were treatment with at least one dose of daptomycin, having initiated and completed daptomycin therapy within the trial timelines, follow-up of at least 30 days after end of treatment, all mandatory information available in hospital files. Written informed consent was waived due to the nature of study design – registry (retrospective, observational). Patients who had received daptomycin as part of a controlled clinical trial were not eligible for this study.
Although seventeen institutions were initially evaluated for participation, only some of them had sufficient time and adequate number of patients who met eligibility criteria (daptomycin had been recently launched in the Brazilian market, on April, 2009). From seven Brazilian institutions that were considered for participation in the study, six collected retrospective clinical data from medical records using a standardized clinical research form (CRF) and protocol (approved by the health authority and Institutional Review Boards). All information collected reflected standard practice in each site and there was no intervention or restriction in clinical practice. Patient data could be recorded into this registry after a minimum of 30 days after the end of daptomycin therapy in order to capture AEs/SAEs. The reasons for premature study drug discontinuation, reason for completion of daptomycin treatment, and antibiotic use after daptomycin treatment were recorded. In cases of multiple infections, investigators entered the type of infection in order of clinical significance (in order of most to least severe): endocarditis, osteomyelitis, bacteremia, other [CNS infection, foreign body/prosthetic infection, metastatic abscess, necrotizing fasciitis, necrotizing infection, surgical/non-surgical antibiotic prophylaxis, septic arthritis and urinary tract infection/pyelonephritis], cSSTI, and uncomplicated skin and soft tissue infection. Safety analysis included all reports of AEs, the severity of which was determined by the investigators. AEs were recorded regardless of their relationship to daptomycin therapy.
The CRFs collected the following information: treatment period, demographics, underlying diseases, pregnancy, neutropenia, antimicrobials, use of other antibiotics and statins, renal function, creatine phosphokinase (CPK) concentrations, diagnosis and details of the current infection, doses of current antibiotic treatment, duration of inpatient and/or outpatient treatment, outcomes, AEs and SAEs occurring between treatment onset and 30 days after last dose of daptomycin, and discharge information.
Statistical analysis was based on pooled data from the individual study sites using the SAS® software version 9.3. All data analyses are descriptive and/or exploratory and consist of tabulations of the collected data. No pre-defined statistical hypotheses were tested.
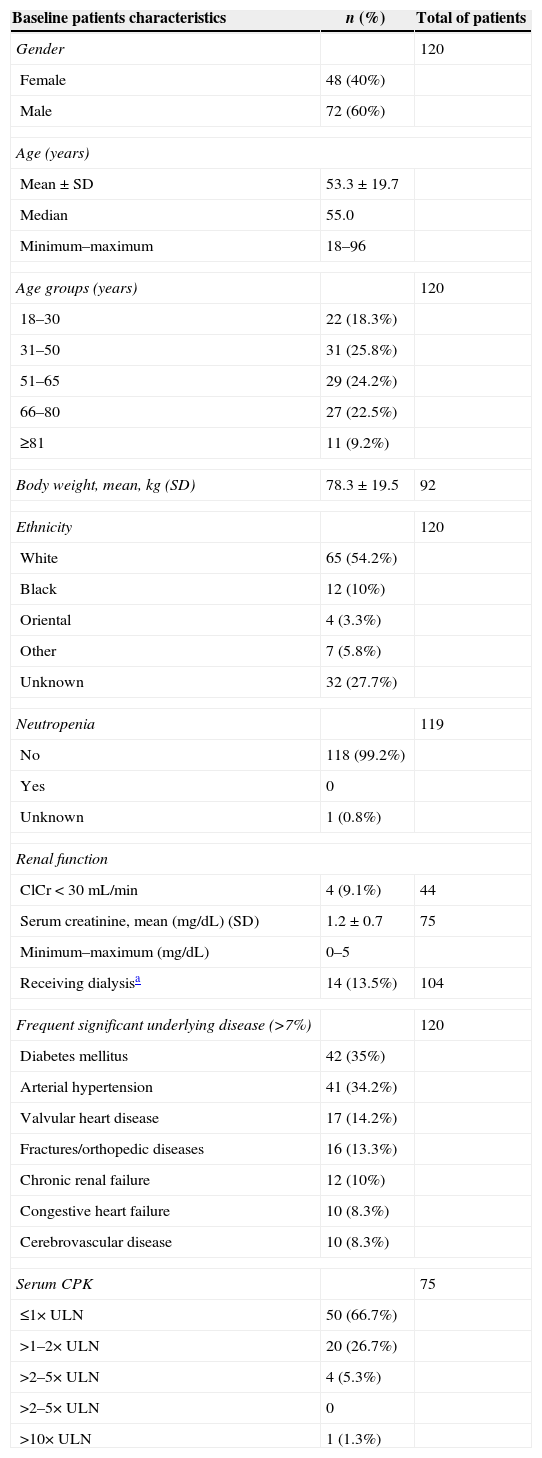
ResultsBaseline patient demographics and clinical characteristicsThe baseline patients’ characteristics at start of daptomycin therapy are described in Table 1, with 38 (31.7%) patients aged ≥65 years. Diabetes mellitus and arterial hypertension were the most common significant underlying diseases, occurring in 35% and 34.2% of the patients, respectively. Other frequent underlying comorbidity included valvular heart disease (14.2%) and fractures/orthopedic diseases (13.3%). Eighteen (15%) patients were considered to have community-acquired infections because they were out of hospital 48h prior to the onset of starting daptomycin therapy. Most patients (82.5%) did not receive HMG-CoA (β-hydroxy-β-methylglutaryl coenzyme A) reductase inhibitor (statin) concomitantly with daptomycin.
Baseline patients’ characteristics at start of daptomycin therapy.
| Baseline patients characteristics | n (%) | Total of patients |
|---|---|---|
| Gender | 120 | |
| Female | 48 (40%) | |
| Male | 72 (60%) | |
| Age (years) | ||
| Mean±SD | 53.3±19.7 | |
| Median | 55.0 | |
| Minimum–maximum | 18–96 | |
| Age groups (years) | 120 | |
| 18–30 | 22 (18.3%) | |
| 31–50 | 31 (25.8%) | |
| 51–65 | 29 (24.2%) | |
| 66–80 | 27 (22.5%) | |
| ≥81 | 11 (9.2%) | |
| Body weight, mean, kg (SD) | 78.3±19.5 | 92 |
| Ethnicity | 120 | |
| White | 65 (54.2%) | |
| Black | 12 (10%) | |
| Oriental | 4 (3.3%) | |
| Other | 7 (5.8%) | |
| Unknown | 32 (27.7%) | |
| Neutropenia | 119 | |
| No | 118 (99.2%) | |
| Yes | 0 | |
| Unknown | 1 (0.8%) | |
| Renal function | ||
| ClCr<30mL/min | 4 (9.1%) | 44 |
| Serum creatinine, mean (mg/dL) (SD) | 1.2±0.7 | 75 |
| Minimum–maximum (mg/dL) | 0–5 | |
| Receiving dialysisa | 14 (13.5%) | 104 |
| Frequent significant underlying disease (>7%) | 120 | |
| Diabetes mellitus | 42 (35%) | |
| Arterial hypertension | 41 (34.2%) | |
| Valvular heart disease | 17 (14.2%) | |
| Fractures/orthopedic diseases | 16 (13.3%) | |
| Chronic renal failure | 12 (10%) | |
| Congestive heart failure | 10 (8.3%) | |
| Cerebrovascular disease | 10 (8.3%) | |
| Serum CPK | 75 | |
| ≤1× ULN | 50 (66.7%) | |
| >1–2× ULN | 20 (26.7%) | |
| >2–5× ULN | 4 (5.3%) | |
| >2–5× ULN | 0 | |
| >10× ULN | 1 (1.3%) | |
SD, standard deviation; ClCr, creatinine clearance; CPK, creatine phosphokinase; ULN, upper limit of normal.
RIE (15.8%), cSSTI-wound (15.0%), catheter-related bacteremia (14.2%) and non-catheter-related bacteremia (10.8%) were the most frequent primary infections for which the patients received daptomycin, but patients with surgical site infections (11.6%), non-complicated SSTI (4.2%), and osteomyelitis (3.3%) also were treated with daptomycin. Lung (21.7%) was the most common site for infection, followed by lower extremity (15%). Secondary infections were reported in 12 (10%) patients, mainly bacteremia (catheter and non-catheter related) and osteomyelitis.
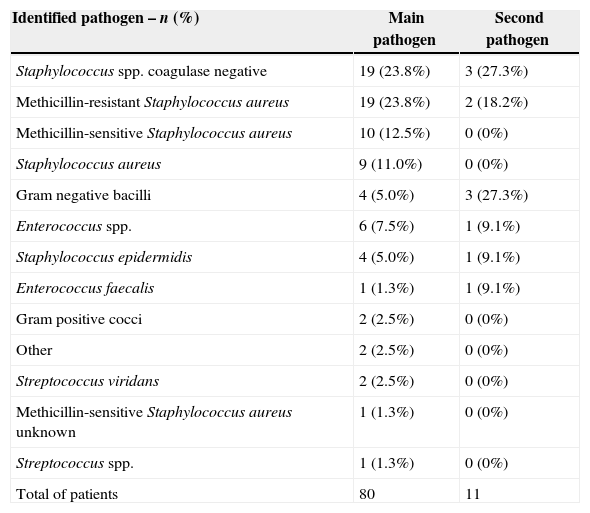
MicrobiologyDaptomycin was used empirically in 76 (63.3%) patients and MRSA was the most commonly suspected pathogen (86.1%), followed by methicillin-sensitive S. aureus (MSSA) (6.9%) and VRE (4.2%). Most cultures (82.5%) (mainly blood, but also deep tissue, needle aspirate, and venous catheter) were obtained prior to or shortly after initiation of daptomycin therapy. Staphylococcus spp. coagulase negative (23.8%), MRSA (23.8%) and MSSA (12.5%) were the most frequently identified pathogens in cultures. The identified pathogens are shown in Table 2.
Identified pathogens as primary infection.
| Identified pathogen – n (%) | Main pathogen | Second pathogen |
|---|---|---|
| Staphylococcus spp. coagulase negative | 19 (23.8%) | 3 (27.3%) |
| Methicillin-resistant Staphylococcus aureus | 19 (23.8%) | 2 (18.2%) |
| Methicillin-sensitive Staphylococcus aureus | 10 (12.5%) | 0 (0%) |
| Staphylococcus aureus | 9 (11.0%) | 0 (0%) |
| Gram negative bacilli | 4 (5.0%) | 3 (27.3%) |
| Enterococcus spp. | 6 (7.5%) | 1 (9.1%) |
| Staphylococcus epidermidis | 4 (5.0%) | 1 (9.1%) |
| Enterococcus faecalis | 1 (1.3%) | 1 (9.1%) |
| Gram positive cocci | 2 (2.5%) | 0 (0%) |
| Other | 2 (2.5%) | 0 (0%) |
| Streptococcus viridans | 2 (2.5%) | 0 (0%) |
| Methicillin-sensitive Staphylococcus aureus unknown | 1 (1.3%) | 0 (0%) |
| Streptococcus spp. | 1 (1.3%) | 0 (0%) |
| Total of patients | 80 | 11 |
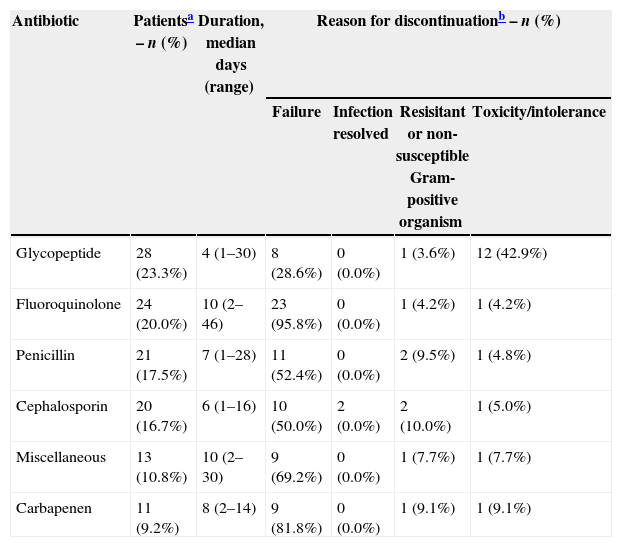
When considering the primary infection, 76 (63.3%) patients had received previous antibiotic therapy. As seen in Table 3, the most frequently used class of antibiotic prior to daptomycin was glycopeptides and treatment failure was the primary reason for discontinuation of previous antibiotic therapy. Eighty-two patients (68.3%) used antibiotics concomitantly with daptomycin: carbapenen (22.5%), miscellaneous (20.8%), penicillin (14.2%), cephalosporin (5.8%), and aminoglycoside (3.3%).
Most frequently (>9%) used antibiotics for the primary infection prior to daptomycin therapy and the reasons for discontinuation.
| Antibiotic | Patientsa – n (%) | Duration, median days (range) | Reason for discontinuationb – n (%) | |||
|---|---|---|---|---|---|---|
| Failure | Infection resolved | Resisitant or non-susceptible Gram-positive organism | Toxicity/intolerance | |||
| Glycopeptide | 28 (23.3%) | 4 (1–30) | 8 (28.6%) | 0 (0.0%) | 1 (3.6%) | 12 (42.9%) |
| Fluoroquinolone | 24 (20.0%) | 10 (2–46) | 23 (95.8%) | 0 (0.0%) | 1 (4.2%) | 1 (4.2%) |
| Penicillin | 21 (17.5%) | 7 (1–28) | 11 (52.4%) | 0 (0.0%) | 2 (9.5%) | 1 (4.8%) |
| Cephalosporin | 20 (16.7%) | 6 (1–16) | 10 (50.0%) | 2 (0.0%) | 2 (10.0%) | 1 (5.0%) |
| Miscellaneous | 13 (10.8%) | 10 (2–30) | 9 (69.2%) | 0 (0.0%) | 1 (7.7%) | 1 (7.7%) |
| Carbapenen | 11 (9.2%) | 8 (2–14) | 9 (81.8%) | 0 (0.0%) | 1 (9.1%) | 1 (9.1%) |
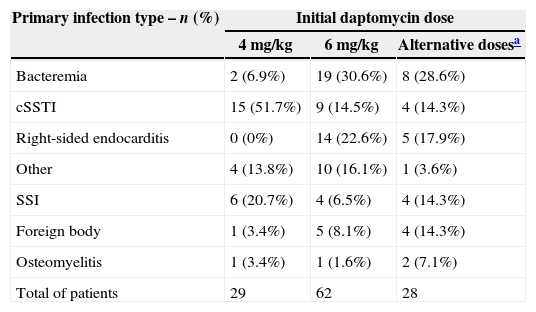
Most common daptomycin dose administered for bacteremia and endocarditis was 6mg/kg and for cSSSI was 4mg/kg, with 28 (23.3%) patients receiving alternative doses (Table 4). Daptomycin was prescribed once a day, in a 30-min infusion, and was the first line therapy in 44 (36.7%) patients.
Daptomycin dosing by primary infection type.
| Primary infection type – n (%) | Initial daptomycin dose | ||
|---|---|---|---|
| 4mg/kg | 6mg/kg | Alternative dosesa | |
| Bacteremia | 2 (6.9%) | 19 (30.6%) | 8 (28.6%) |
| cSSTI | 15 (51.7%) | 9 (14.5%) | 4 (14.3%) |
| Right-sided endocarditis | 0 (0%) | 14 (22.6%) | 5 (17.9%) |
| Other | 4 (13.8%) | 10 (16.1%) | 1 (3.6%) |
| SSI | 6 (20.7%) | 4 (6.5%) | 4 (14.3%) |
| Foreign body | 1 (3.4%) | 5 (8.1%) | 4 (14.3%) |
| Osteomyelitis | 1 (3.4%) | 1 (1.6%) | 2 (7.1%) |
| Total of patients | 29 | 62 | 28 |
cSSTI, complicated skin and soft tissue infections; SSI, surgical site infection.
In the inpatient setting, the median duration of daptomycin therapy was 14 days (range 2–82 days), usually with concomitant antibiotics, with 51 (42.9%) patients receiving daptomycin in an ICU for a median duration of seven days (range 1–21 days). For patients treated as outpatient (n=8), the median duration of therapy was 30 days (range 5–85 days) and no concomitant antibiotic was used in 93.5% of the patients.
The main reason for daptomycin discontinuation was successful completion of therapy (75.8%), switched therapy (11.7%) and treatment failure (4.2%).
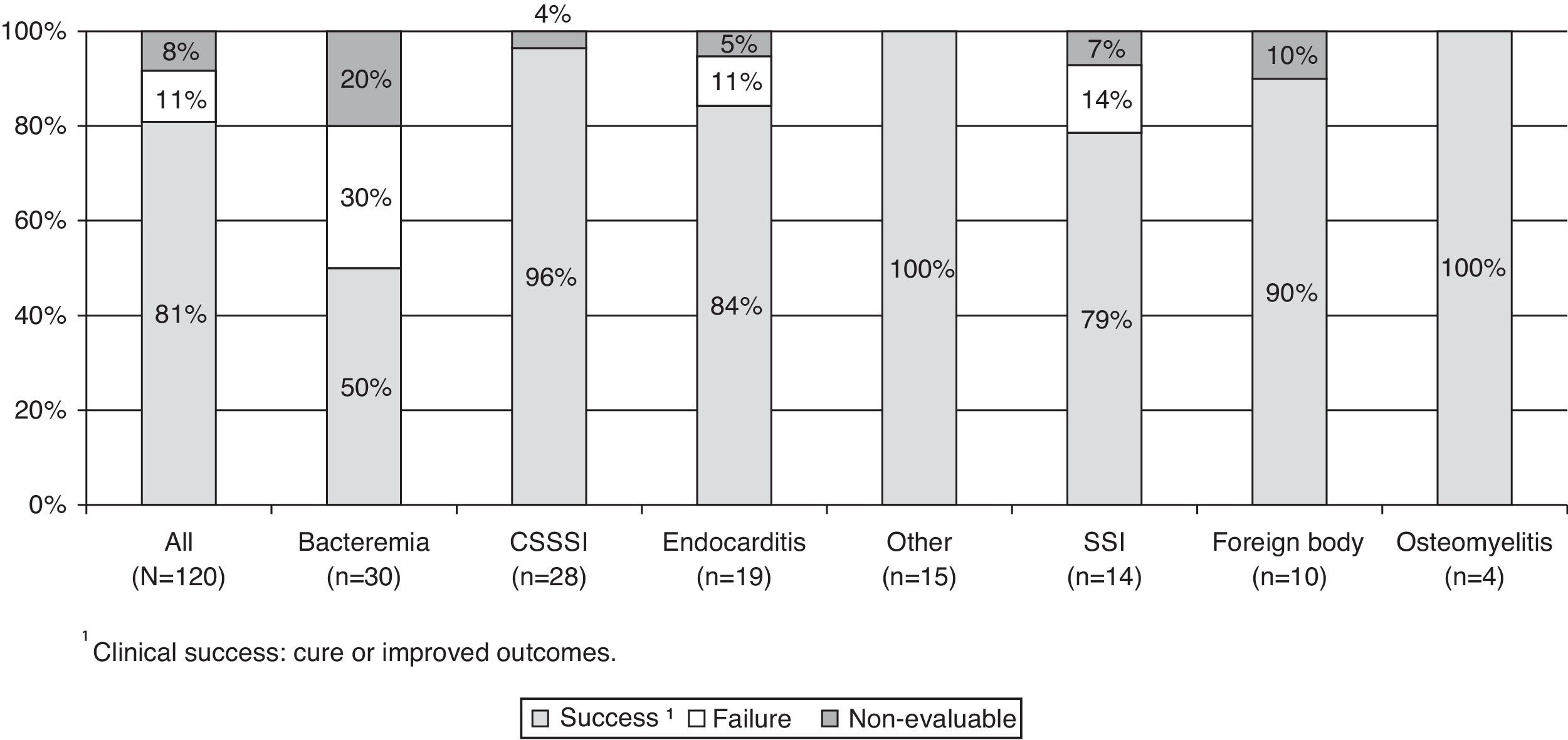
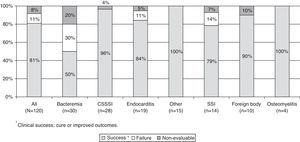
Clinical outcomeThe patients showed clinical improvement after a median of two days (range 1–29) after the start of daptomycin therapy. The rate of clinical success (cure and improvement) was 80.8% and the overall rate of treatment failure was 10.8%. All sites of infections, except bacteremia, achieved high rates of cure (Fig. 1).
Safety and tolerabilityThe safety profile of daptomycin was favorable and was assessed in all 120 patients included in this trial. Seven patients (5.8%) had AE and 5 (4.2%) had SAEs like death (n=4) or life threatening situations (n=1). Investigators classified 2.5% (n=3) of the AEs as possibly related to daptomycin, and permanently discontinued the treatment. The median AE onset and resolution was eight days (range 1–21) and 12 days (range 2–51), respectively. The AEs probably related to daptomycin were ventricular asystole, upper gastrointestinal hemorrhage and increased CPK.
SAEs were, with one event of each, endocarditis, hemorrhagic shock, embolism, ventricular asystole, hypoxic–ischemic encephalopathy, metabolic encephalopathy, convulsion, acute renal failure, systemic candidiasis, cerebral ischemia, gastroduodenitis, device related infection, stress ulcer, and upper gastrointestinal hemorrhage; and septic shock (2 events).
There was no change in renal function/status and no neutropenia during daptomycin therapy. Serum CPK was measured at baseline in 75 (62.5%) patients and during the course of daptomycin therapy in 73 (61.3%) patients. Serum CPK levels measured at baseline are shown in Table 1. During therapy, no patient had CPK elevations above 10× ULN and one had a CPK elevation between 5 and 10× ULN.
DiscussionThis trial reflects the experience of six Brazilian sites with daptomycin, which is used to treat cSSTI due to Gram-positive bacteria and SAB (S. aureus bacteremia), including RIE, but also surgical site infections, osteomyelitis and non-complicated SSTI, suggesting that there is an unmet medical need not included in label indications. Daptomycin is being used to treat other infections caused by other Gram-positive species, as cancer9,10 and orthopedic-related infections.11–14 Despite most patients being under 65 years (68.3%), they had several comorbidities, most of them had been hospitalized 48h before the start daptomycin and 63.3% had received previous antibiotic therapy, which may have favored the emergence of infections with drug-resistant pathogens as MRSA, MR-CoNS and VRE. Older patients (>65 years) had good tolerability and clinical outcomes with daptomycin, consistent with the results of other studies.15
As first-line treatment, the antibiotics of choice were glycopeptides (vancomycin), fluoroquinolone, penicillin, cephalosporin, or carbapenen. Daptomycin was used as second-line treatment in 63.3% of the patients and the switch to daptomycin was consequence of treatment failure, resistant or non-susceptible Gram-positive bacteria or toxicity/intolerance. The main cause for discontinuation of vancomycin was toxicity/intolerance and for other antibiotics was treatment failure. So, daptomycin has an important role as first-line therapy for Gram-positive infections.16
Daptomycin 4mg/kg intravenously every 24h for 7–14 days is the gold standard for cSSTI17 and 6mg/kg intravenously every 24h for bacteremia and RIE.18 However, 23.3% of the patients received alternative dosages, mainly between 7.0 and 9.9mg/kg/day, regardless of the severity of the infection. In clinical practice, according to infection severity, there is a trend to use higher doses of daptomycin, with good efficacy without concerns about toxicity.19 Concomitant administration of other antibiotic agents with daptomycin was common practice and aminoglycosides were among the most frequent with concomitant use in 15% of patients.20 In Brazil the most frequently used was carbapenen.
The median treatment duration of inpatient therapy was 14 days (range 2–82 days) in this registry. In a similar trial, for those patients receiving prolonged therapy with daptomycin, there is no report of increased incidence in AEs.20 As there are relatively few treatment options available for resistant Gram positive infections that can be used for long periods without an increased risk of AEs, daptomycin might, therefore, represent a useful option for the treatment of chronic complicated infections where extended duration of therapy is required, such as osteoarticular or endovascular infections.20
Although few Brazilian patients have received daptomycin therapy as outpatient, data indicate that outpatient parenteral daptomycin is a suitable agent in cases of selected Gram positive bacterial infections. After a relatively brief hospitalization, the majority of patients can complete at least three fourths of total treatment duration outside of the hospital, receiving 2-min daptomycin injections.21
The clinical outcome was favorable for daptomycin, as patients showed a clinical improvement after a median of two days, the overall clinical success rate was 80.8% and the overall rate of treatment failure was 10.8%. Only 11.7% of the patients switched daptomycin to another antibiotic and 4.2% stopped due to treatment failure. Based on the results presented, one can conclude that, unlike glycopeptides, daptomycin has similar efficacy against both MRSA and MSSA, making it an important option for empirical therapy of suspected S. aureus infections, regardless of methicillin resistance risk.17,18
The safety profile of daptomycin was favorable. The AEs probably related to daptomycin were ventricular asystole, upper gastrointestinal hemorrhage and increased CPK. Daptomycin had been associated with reversible CPK elevation and skeletal muscle toxicity before the optimization of the dosing interval to a once-daily regimen. In clinical trials with once-daily dosing, daptomycin-associated CPK elevations were demonstrated in 7% of patients receiving the 6mg/kg dose, and was not observed at the 4mg/kg dose.25,26 The safety and tolerability profile and low overall rates of clinical failure were similar to the rates reported around the world.20,22,23
Since the use of statins could increase plasma CPK levels during therapy with daptomycin and statins are known to be associated with myopathy, it is recommended to avoid this association during daptomycin treatment.8 In our registry, most patients (82.5%) did not receive a HMG-CoA reductase inhibitor with daptomycin.
Although there was no change in renal function/status during daptomycin therapy, it is important to remember that the dosing interval in patients with severe renal impairment (ClCr<30mL/min) with or without dialysis should be adjusted to q48h for both approved doses.8,24,25
Many publications about CORE (2003–2008) and EU-CORE (2006–2011) results have been published since the first report in 2006. This Brazilian report is similar to an European report published in 2011 showing data from the first 2.5 years old daptomycin use.26 In this European report, 1127 patients were included in the registry, the most frequent infection diagnosed was cSSTI (33%), bacteremia (22%), endocarditis (12%) and osteomyelitis (6%). Daptomycin was empirically used in 53% of the patients. S. aureus was the most frequently isolated pathogen (34%), with 52% resistant to methicillin. The overall clinical success rate was 79%, with a clinical failure rate of <10% for all infections.
There are other 35 reports indexed about these 2 studies, many of them showing results from specific populations, such as, diabetic patients,27 patients with chronic renal failure,28 critical care patients,23 patients with hematological malignancies,29 surgical site infections,30 all presenting an overall success rate between 70% and 90%.
As the registry is retrospective, non-comparative, non-blinded and non-randomized and clinical outcomes are judged by the attending clinician, this trial has some limitations related to data collection. However, data are important because reflect the clinical practice.
In conclusion, daptomycin has some activity against a broad range of Gram positive pathogens, including organisms that are resistant to methicillin, vancomycin, and other currently available agents. Considering that the most frequently treated infections were RIE, cSSTI or bacteremia, for which the likelihood of vancomycin failure is high, daptomycin could be considered a good option for these indications. Therefore, this first-in-class cyclic lipopeptide antibiotic could be considered an important option for Brazilian patients.
Financial supportThis study was supported by a grant from Novartis.
Conflicts of interestThe authors declare no conflicts of interest.