HIV patients infected through injected drug use have poorer prognosis than other groups. We evaluated the hematological alterations and rates of co-infections in injected drug use patients with AIDS. Injected drug use patients were younger, predominantly of male gender, and presented lower CD4, total lymphocyte, and platelet counts, but not neutrophil count, than control group. Injected drug use patients had a higher rate of hepatitis C and mycobacteria infection. Furthermore, all injected drug use patients with hemoglobin <10.0gdL−1 and lymphocyte <1000μL−1 had CD4 count lower than 100μL−1. In conclusion, HIV-infected injected drug use patients constitute a special group of patients, and hemoglobin concentration and lymphocyte count can be used as surrogate markers for disease severity.
Injected drug use is a risk factor for HIV infection and has been associated with poor outcome.1 Progression to AIDS and mortality rate are substantially higher in injected drug users (IDU).2,3 Many aspects have been associated to this fact: adverse socioeconomic conditions, psychiatric disorders, poor compliance with treatment, and medical restrictions in prescribing antiretroviral therapy, due to concerns of selecting of multidrug-resistant HIV.3 Hepatitis co-infection is commoner in this population.2 HIV infection and AIDS are associated with a wide spectrum of hematological abnormalities.4,5 Blood cytopenias can be found in most patients during disease evolution.4 Their etiology is multifactorial and include the direct effect of HIV, opportunistic infections and malignancies, or therapies used in the management of AIDS.4 Progressive depletion of CD4 lymphocytes is the hallmark of advancing HIV disease and leads to the immunosuppression.4 This retrospective cross-sectional study evaluated the hematological particularities of HIV-infected IDU patients and rates of hepatitis and mycobacteria co-infections. Furthermore, we intended to correlate these alterations with CD4 count, and establish a surrogate marker of disease severity combining the results of hemoglobin concentration (Hb) and lymphocyte count.
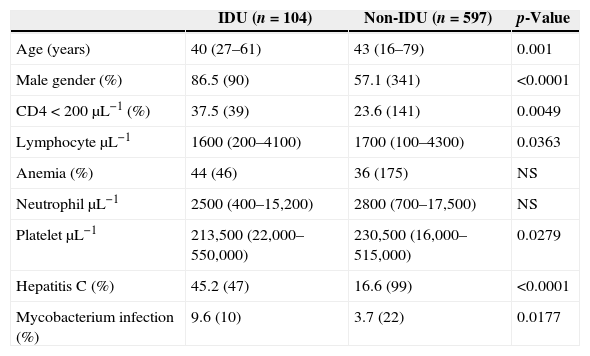
Materials and methodsWe reviewed the data of 701 consecutive HIV-infected patients (≥16 years) followed at our institution, of which 14.8% (104) reported injected drug use (Table 1). The parameters recorded were HIV-viral load, Hb, CD4, lymphocyte, neutrophil, and platelet counts. Blood cell and CD4 counts, and HIV viral load quantification were performed as previously described.5 Anemia was defined as Hb<12.0gdL−1 for women and <14.0gdL−1 for men. Statistical analyses were performed using SAS® (version 9.1).
Patients’ characteristics.
| IDU (n=104) | Non-IDU (n=597) | p-Value | |
|---|---|---|---|
| Age (years) | 40 (27–61) | 43 (16–79) | 0.001 |
| Male gender (%) | 86.5 (90) | 57.1 (341) | <0.0001 |
| CD4<200μL−1 (%) | 37.5 (39) | 23.6 (141) | 0.0049 |
| LymphocyteμL−1 | 1600 (200–4100) | 1700 (100–4300) | 0.0363 |
| Anemia (%) | 44 (46) | 36 (175) | NS |
| NeutrophilμL−1 | 2500 (400–15,200) | 2800 (700–17,500) | NS |
| PlateletμL−1 | 213,500 (22,000–550,000) | 230,500 (16,000–515,000) | 0.0279 |
| Hepatitis C (%) | 45.2 (47) | 16.6 (99) | <0.0001 |
| Mycobacterium infection (%) | 9.6 (10) | 3.7 (22) | 0.0177 |
IDU, injection drug user; NS, not significant.
Values expressed as percentage (absolute number) or median (range).
This study was approved by the institution Ethics Committee.
ResultsMedian ages of IDU and non-IDU groups were 40 (27–61) and 43 (16–79) years, respectively (p=0.001). Men comprised 86.5% (90/104) of IDU patients and 57.1% (341/597) of non-IDU patients (p<0.0001). CD4 count was lower among IDU than non-IDU patients. CD4 was <200dL−1 in 37.5% (39/104) and 23.6% (141/597) of IDU and non-IDU patients, respectively (p=0.0049). Medians of lymphocyte counts were 1600μL−1 (200–4100μL−1) and 1700μL−1 (100–4300μL−1) for IDU and non-IDU groups, respectively (p=0.0363). We did not find a significant association between viral load and use of injected drugs (p=0.2192). A total of 37.7% (264/701) of the patients had anemia at the moment of analysis, 44% (46/104) among IDU group and 36% (175/432) among non-IDU group (p=0.15). Medians of Hb were 11.8gdL−1 (6.4–15.4gdL−1) and 12.8gdL−1 (6.7–15.9gdL−1) for IDU and non-IDU women, respectively (p=0.2587); and 14.3 (6.7–18.7gdL−1) and 14.4gdL−1 (6.4–20gdL−1) for IDU and non-IDU men, respectively (p=0.6664). Furthermore, all IDU individuals with Hb<10gdL−1 and lymphocyte <1000μL−1 (n=10) presented a CD4<100dL−1 (specificity 100%, sensitivity 29.4%, positive predictive value (PPV) 100% and negative predictive value (NPV) 67.3%). Among the non-IDU group, 81.8% (18/22) of the individuals with Hb<10gdL−1 and lymphocyte <1000μL−1 presented CD4<200dL−1 (specificity 99.12%, sensitivity 12.7%, PPV 81.8% and NPV 75.7%). Medians of neutrophil counts were 2500μL−1 (400–15,200μL−1) and 2800μL−1 (700–17,500μL−1) for IDU and non-IDU groups, respectively (p=0.1674). Medians of platelet counts were 213,500μL−1 (22,000–550,000μL−1) and 230,500μL−1 (16,000–515,000μL−1) for IDU and non-IDU groups, respectively (p=0.0279). After excluding the patients with hepatitis C (HCV), we did not find a significant association between platelet number and injected drug use (p=0.6868). A total of 45.2% (47/104) of the IDU group had HCV infection, diagnosed by Elisa and confirmed by PCR, in contrast to 11% among the non-IDU group (p<0.0001). Chronic hepatitis B was present in 9.6% of the IDU and 4.8% of the non-IDU (p=0.0622) groups. A total of 24% (25/104) of IDU and 16.6% (99/597) of non-IDU groups reported poor adherence to treatment (p=0.071). Mycobacteria infection was diagnosed (8 in sputum, 1 in gastric fluid and 1 by lymph node biopsy) in 9.6% (10/104; 6 tuberculosis) among IDU patients and in only 3.7% (22/597; 13 tuberculosis) in the non-IDU (p=0.0177) group. We did not find any significant association between injected drug use and other opportunistic infections (data not shown). Mortality was similar in the IDU (1.9%; 2/104) and non-IDU (4.2%; 25/597) groups after 1 year of follow-up (p=0.4073).
DiscussionIn this study, the IDU group comprised a higher percentage of men than non-IDU patients, a finding that is in accordance with a previous report.3 Moreover, the IDU group was younger, which is also in line with a previous report6 but not with another one.3 CD4 cells are the main target of HIV infection and their reduced count is associated with disease progression. We found a lower CD4 count in the IDU group compared with the non-IDU group, despite having similar viral loads. Dronda et al.6 have shown long-term poorer recovery of CD4 cells despite successful virological suppression in IDU patients. Anemia is the commonest hematological abnormality in HIV-infected patients and its incidence is associated with disease severity and mortality.7,8 Anemia was not higher among IDU than non-IDU patients, in disagreement with data shown by others.9 Besides anemia, thrombocytopenia is often observed in HIV-infected patients. Approximately 40% of HIV patients present thrombocytopenia during the course of their illness.10 As with other HIV-associated cytopenias, the etiology of thrombocytopenia is multifactorial. Megakaryocytes express CD411 and CXCR-412 and, thereby, are targets for HIV. Besides, expression of viral proteins or altered antigens on the cell membrane make infected megakaryocytes susceptible to immune attack.13 Furthermore, HCV infection, commonly found in HIV-infected patients especially among IDU, is a well-known cause of thrombocytopenia.14 In this study the percentage of HCV among IDU patients was inferior than previously reported.6,15 We found a higher frequency of thrombocytopenia in IDU patients, but this finding was observed only in HCV infected patients. Tuberculosis is an endemic infection in Brazil, and its incidence, along with non-tuberculous mycobacterial infections, is increased in HIV patients, especially in the IDU group, a finding that is in accordance with other studies.16 Furthermore, the proposal of an inexpensive surrogate marker for AIDS severity, such as hemoglobin concentration and lymphocyte count, could be a useful tool for evaluations of AIDS severity in developing countries, where the high prevalence of HIV and low financial resources limit adequate disease monitoring. In conclusion, HIV-infected IDU patients tend to present a more severe disease, as demonstrated by a higher rate of thrombocytopenia and more profound immunosuppression.
Conflicts of interestThe authors declare no conflicts of interest.