For clinicians, a practical bedside tool for severity assessment and prognosis of patients with Clostridium difficile infection is a highly desirable unmet medical need.
SettingTwo general teaching hospitals in northeast Mexico.
PopulationAdult patients with C. difficile infection.
MethodsProspective observational study.
ResultsPatients included had a median of 48 years of age, 54% of male gender and an average of 24.3 days length of hospital stay. Third generation cephalosporins were the antibiotics most commonly used prior to C. difficile infection diagnosis. Patients diagnosed with C. difficile infection had a median ATLAS score of 4 and 56.7% of the subjects had a score between 4 and 7 points. Patients with a score of 8 through 10 points had 100% mortality.
ConclusionThe ATLAS score is a potentially useful tool for the routine evaluation of patients at the time of C. difficile infection diagnosis. At 30 days post-diagnosis, patients with a score of ≤3 points had 100% survival while all of those with scores ≥8 died. Patients with scores between 4 and 7 points had a greater probability of colectomy with an overall cure rate of 70.1%.
Clostridium difficile infection (CDI) is a serious threat in the hospital setting and it is now one of the leading causes of healthcare-associated infection.1,2 Serious complications such as sepsis, toxic megacolon, need for colectomy, and death are associated with CDI especially in those patients infected by hypervirulent strains.3
The need of a proper instrument for severity assessment has been previously studied with various results.4–6 For clinicians, a practical bedside tool for severity assessment and prognosis is a highly desirable unmet medical need. One of the most practical bedside severity scoring systems is the ATLAS system that has recently been validated7 based upon two large clinical trials comparing vancomycin and fidaxomicin (Table 1). We sought to apply the ATLAS score in two Mexican teaching hospitals and compare some of our findings to the published data.
MethodsThis prospective study was performed from November 2011 to March 2014. The study was carried out in two teaching hospitals from the northeastern region of Mexico: the University Hospital “Dr. José Eleuterio González” tertiary care hospital with 450 beds in Monterrey and Hospital Christus Muguerza Alta Especialidad a private teaching hospital with 200 beds. Patients were included in the study if they were hospitalized for more than 48h and had more than three bowel movements in the previous 24h (Bristol scale 6–7), and no other cause of diarrhea plus a positive C. difficile toxin assay (Immunocard toxin AB, Meridian Bioscience Inc.); and/or a colonoscopy showing pseudomembranous colitis. All cases had to have a positive stool culture for toxigenic C. difficile. Clinical cure was defined as the absence of diarrhea or inflammatory response at the end of treatment and no recurrence 28 days after the end of treatment. For comparison of dichotomous variables we applied Fishers exact test or Chi-Square test. A p-value of ≤0.05 was considered statistically significant.
ResultsA total of 102 patients were included with a median of 48 years of age and a slight predominance of males (54%). They had an average length of hospital stay (LOS) of 24.3 days, 14.6 days of antibiotic therapy, and 5.3 days of diarrhea prior to CDI diagnosis. Third generation cephalosporins were the antibiotics most commonly used prior to CDI diagnosis (47.0%) followed by fluoroquinolones (33.3%) and clindamycin (23.5%). There were 3.9% (4/102) total colectomies and 30-day all-cause mortality was 17.6% (18/102) (Table 2).
Characteristics and outcome of the study population of CDI patients.
| Demographic characteristics | n=102 |
| Mean age | 49.79 (15–97) |
| Male gender | 55 (53.92%) |
| Risk factors | |
| Average BMI | 25.69 |
| Total LOS (days) | 24.38 |
| LOS before CDI diagnosis | 11.89 |
| LOS after CDI diagnosis | 12.72 |
| Mean number of antibiotics use before CDI | 2.35 |
| Average antibiotic days before CDI | 14.61 |
| Postsurgical diagnosis | 46 (45.9%) |
| Clinical characteristics | |
| Average number of bowel movements | 5.59 |
| Days with diarrhea before diagnosis of CDI | 5.38 |
| Average leukocyte count | 13,897 |
| Fever | 55 (53.9%) |
| Outcome | |
| ≤28 day mortality | 18 (17.6%) |
| >28 day mortality | 5 (4.9%) |
| Median ATLAS score | 4 |
| Colectomy | 4 (3.9%) |
| Prior antibiotics used | |
| Third generation cephalosporins | 48 (47.05%) |
| Fluoroquinolones | 34 (33.3%) |
| Carbapenems | 26 (25.4%) |
| Clindamycin | 24 (23.52%) |
| Antibiotics used for the treatment of CDI | |
| Metronidazole | 58 (56.86%) |
| Vancomycin | 7 (6.86%) |
| Metronidazole+vancomycin | 37 (36.27%) |
CDI, Clostridium difficile infection; LOS, length of stay.
Metronidazole was the treatment for 58/102 patients (56.8%), oral vancomycin in 7/102 (6.8%), and a combination of metronidazole PO/IV and vancomycin PO in 37/102 patients (36.2%). Mortality was statistically different between patients with a serum albumin of ≤2.5g/dL compared with those with higher albumin levels [18 (40%) vs. 4 (7.0%) p<0.0001].
The day on which the patients were diagnosed with CDI, the distribution of ATLAS scores among this cohort can be seen in Table 2. All four patients with a score of eight or greater died during the 30 days after the diagnosis of CDI: three of them as a result of septic complications of CDI and the remaining patient because of cancer related complications. There were four colectomies performed as a therapeutic measure for CDI; two patients with a score of 5 points each, one with a score of 6 and another with a score of 7 all of whom survived. In addition, no deaths or colectomies occurred among the patients having an ATLAS score of 2 points or less.
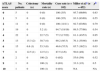
DiscussionA simple bedside scoring system for evaluating severity and prognosis in CDI patients continues to be an important unmet medical need. The ATLAS score is one of the simplest tools for the prediction of cure in patients with CDI. The score was validated in a large database7 that was extrapolated from two CDI studies8,9 comparing vancomycin and fidaxomicin for the treatment of CDI. In the current study we applied the ATLAS scoring system in a cohort of patients diagnosed with CDI and followed them prospectively. The cure rate was similar to the previous description7 of this scoring system with the exception of the group with a score of 6 points, in which our group had a lower cure rate (Table 3). In this prospective observational study, we did not intervene or alter the treatments since those variables were decided by the treating physician. Although the study did not interfere with the treatment decision, the majority of patients with severe disease received vancomycin as suggested by current guidelines and other studies.10,11 None of the patients were treated with fidaxomicin since this medication was not available in Mexico at the time of the study. Several severity scores have been applied in patients with CDI with sensitivities ranging from 63.2% to 84.2%, and specificities ranging from 59.4% to 93.9%.12 The scoring system used in our study was originally applied as a predictor of cure but in the current study we additionally correlated the score with the need for colectomy and mortality rate, thus enhancing its utility.
Comparison between colectomy, 28-day mortality and cure rates using the ATLAS score in 2 different groups.
| ATLAS score | No. patients | Colectomy (n) | Mortality rate (n) | Cure rate (n=102) | Miller et al.a (n=452) | pb |
|---|---|---|---|---|---|---|
| 0 | 5 | 0 | 0 (0) | 100 (5/5) | 95.7 (66/69) | 0.63 |
| 1 | 5 | 0 | 0 (0) | 100 (5/5) | 93.3 (83/89) | 0.55 |
| 2 | 11 | 0 | 0 (0) | 100 (11/11) | 92.7 (63/68) | 0.79 |
| 3 | 19 | 0 | 5.2 (1) | 94.7 (18/19) | 89.5 (77/86) | 0.54 |
| 4 | 22 | 0 | 22.7 (5) | 77.2 (17/22) | 81.1 (43/53) | 0.95 |
| 5 | 13 | 15.3 (2) | 30.7 (4) | 69.2 (9/13) | 76.1 (35/46) | 0.89 |
| 6 | 15 | 6.6 (1) | 53.3 (8) | 46.6 (7/15) | 85.7 (18/21) | 0.03 |
| 7 | 8 | 12.5 (1) | 12.5 (1) | 87.5 (1/8) | 50.0 (4/8) | 0.28 |
| 8 | 2 | 0 | 100 (2) | 0 (0/2) | 55.6 (5/9) | 0.52 |
| 9 | 1 | 0 | 100 (1) | 0 (0/1) | 33.3 (1/3) | 0.5 |
| 10 | 1 | 0 | 100 (1) | 0 (0/1) | NA | NA |
The decision for performing a total colectomy was evaluated by the treating medical staff and was not controlled by our study investigators. In the current study the four patients with an ATLAS score of ≥8 died without a colectomy. This could be a reflection of their severe clinical condition and the reluctance to refer them for surgery, or a direct consequence of the presence of fulminant colitis and/or sepsis due to the CDI.
ConclusionOur study evaluated the ATLAS score in a teaching hospital scenario finding it a useful tool for routine evaluation of patients with CDI. Those patients with a score of ≤3 points had an excellent prognosis while those who had scores between 4 and 7 points had a greater probability of having a colectomy. Patients with scores ≥8 had 100% mortality, but it is unclear if that was due to the severity of their colitis or because an early enough colectomy was not performed.
In summary, the ATLAS score at the time of CDI diagnosis in our patient population can be used to predict survival and the need for consideration of colectomy. Additionally, it can aid clinicians in choosing which patients to monitor closely and to intervene in order to prevent or treat CDI-related complications, morbidity and mortality.
Conflicts of interestThe authors declare no conflicts of interest.