In recent years, the use of outpatient parenteral antimicrobial therapy (OPAT) has increased, resulting in the need to ensure its rational and adequate utilization. This article describes the implementation of an antimicrobial stewardship program in the OPAT setting by a Health Maintenance Organization (HMO) and its results.
MethodAn infectious disease (ID) physician made routine assessments of all home care parenteral antimicrobial requests from February to December 2019. Information on diagnosis, renal function, weight, previous antimicrobials, and microbiology were gathered during remote evaluations. Prescription changes recommended by the ID specialist were not mandatory, but implemented by the primary provider as accepted. Antibiotic consumption data was analyzed from January 2018 to December 2019. An active screening was conducted for treatment failures: two or more treatment course requirements, or death within 15 days of the evaluation were reexamined.
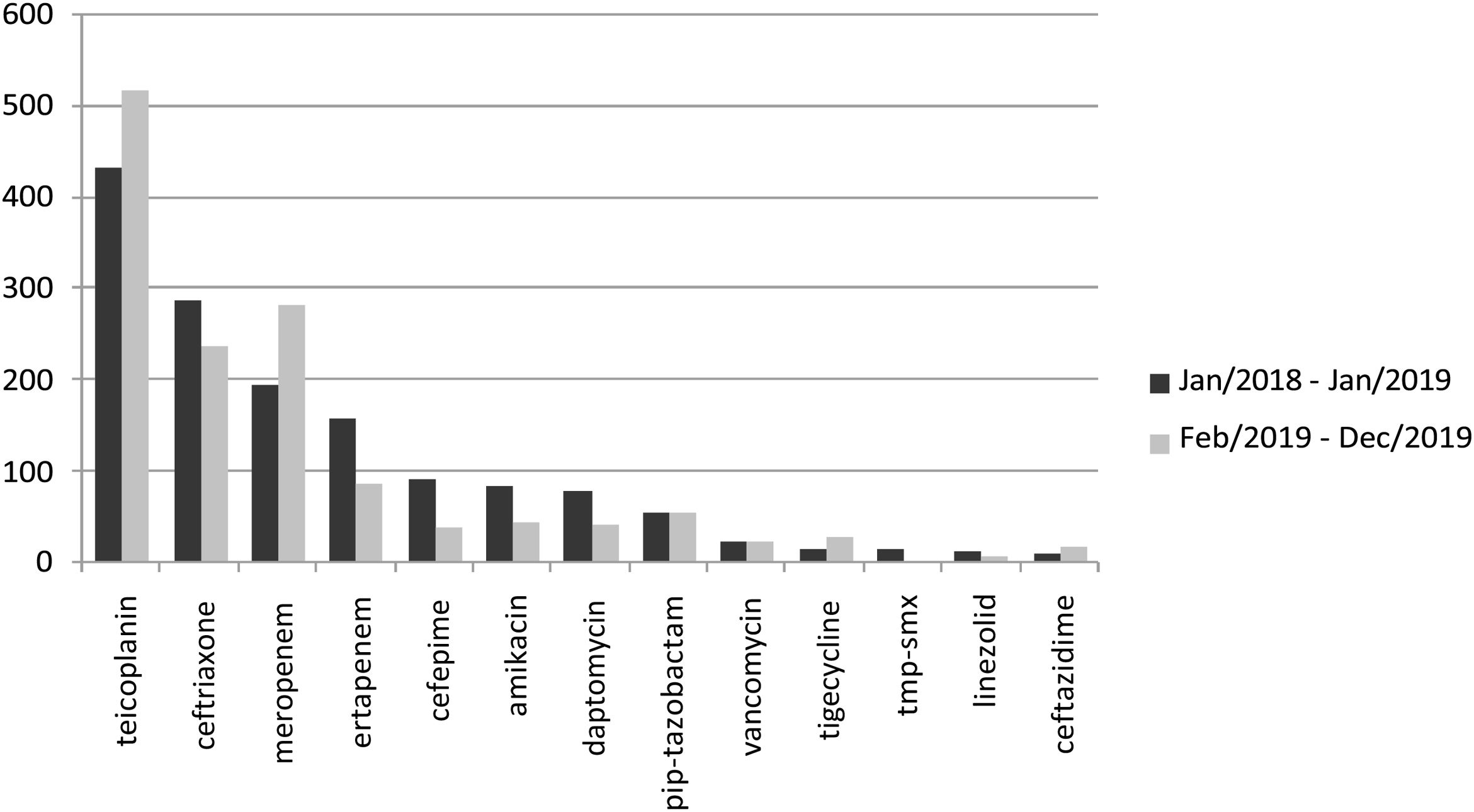
ResultsA total of 506 antimicrobial requests were assessed. The most frequent diagnoses were urinary tract infection, pneumonia, and orthopedic surgical site infection. Six percent of evaluations were not completed due to insufficient information and 12% were requests by the primary physician for initial antimicrobial guidance. Of the 416 completed prescriptions evaluations, 58% had suggested changes, including different antimicrobials (40%), treatment duration (25%), and route of administration (23%). There was an increase in use of teicoplanin and meropenem, and a decrease in ceftriaxone, ertapenem, cefepime, amikacin and daptomycin use. The HMO’s overall parenteral antimicrobial outpatient consumption, which had shown an upward trend over the previous year, decreased after program initiation. No major adverse results were detected in patients’ clinical outcomes; two treatment failures were detected and promptly corrected; no deaths attributed to antibiotic changes were detected.
ConclusionOutpatient antimicrobial stewardship, through remote assessment by an ID specialist, was effective and safe in the OPAT setting.
In recent decades, outpatient parenteral antimicrobial therapy (OPAT) has been used to reduce length of hospital stay,1 and medical associations have made efforts to establish safe criteria for this procedure.2 However, increase in bacterial multidrug resistance and decrease in oral antibiotic options have caused a rapid and massive escalation of home parenteral antibiotic therapy. However, it is necessary to ensure adequate use of this resource, in order to avoid unnecessary increase in costs and risks.3,4 Management strategies must be developed according to the resources available and needs of the health service.
ObjectiveThe aim of the present study was to describe the experience of an antimicrobial stewardship program in the home care setting by a Brazilian Health Maintenance Organization (HMO), including the steps involved to implement the program and results.
MethodThis was a retrospective study of the results of an antimicrobial stewardship activity implemented by a HMO, over the course of 11 months, compared to the year prior to program initiation.
In February 2019, an infectious disease (ID) physician was assigned to evaluate the use of parenteral antimicrobials requested to the outpatient clinic pharmacy. Requests were from both chronic patients with comorbidities followed at the outpatient clinic and from patients discharged from HMO-owned hospitals or an associated network of private hospitals.
The results include all antimicrobial evaluations carried out between February and December 2019. In July 2019, after a considerable increase in the number of members, a separate antimicrobial stewardship program was created, to specifically supply the demands of the HMO’s largest hospital. This other antimicrobial stewardship program followed its own method and its results were not included in the present study.
Evaluations were conducted remotely, using e-mail, telephone, and a messaging application, at any time of the day. A smaller fraction of patients was evaluated during an outpatient ID consult, if necessary.
Antimicrobial evaluations could be requested both directly by prescribing physicians (from hospitals and outpatient clinics) and by nurses who intermediated the contact between hospitals and home care services. Information on diagnoses, clinical evolution, renal function, weight, previous use of antimicrobials, clinical status and microbiology data were provided. The ID physician evaluated each case and issued their recommendation back to the outpatient clinic or discharging hospital, to be considered by the prescribing physician.
The recommendations were made in the form of consultations, without prohibiting the use of the initially prescribed antimicrobial, and aiming to deepen the discussion of evidence-based criteria for each case. Recommendations were based primarily on two reputable academic clinical manuals, both routinely used in clinical practice in the city of São Paulo:
-
Guia de utilização de anti-infecciosos e recomendações para a prevenção de infecções relacionadas a assistência à saúde 2018–2020. (Anti-infective agents use guidelines and recommendations to prevent healthcare-associated infections 2018–2020). Coordination Anna Sara S. Levin et al. Published by Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, 2018.5
-
The Sanford Guide to Antimicrobial Therapy 2019. David N. Gilbert et al. Published by Antimicrobial Therapy Inc., 2019.6
When additional resources were required, the ID physician consulted well-established medical websites, such as UpToDate and PubMed. The ID physician adopted the minimum interference principle, with recommendations for drug substitution, dosage, route of administration, or treatment duration made only when any of the following problems were identified:
- •
Inconsistency between the requested antimicrobial and culture/antibiogram data;
- •
Availability of an oral antimicrobial equivalent to the prescribed parenteral agent, in the absence of any contraindication to oral treatment;
- •
Patient at high risk for drug toxicity;
- •
Treatment duration outside usual standards for the identified source of infection, without justification;
- •
Cost of requested antimicrobial significantly higher than other equally effective options.
The effect of antimicrobial stewardship could be seen on the antimicrobial dispensing data provided by the home care pharmacy.
To identify possible negative impacts of this stewardship, all patients were screened for the need for two or more prescription evaluations. Each case was analyzed as to whether subsequent consultations were due to problems with the prior treatment modification or not. Deaths occurring within 15 days after the evaluation were also assessed to investigate whether the suggested antimicrobial changes could be associated with the unfavorable outcome.
All data were recorded and analyzed in Excel. The statistical analysis was primarily descriptive.
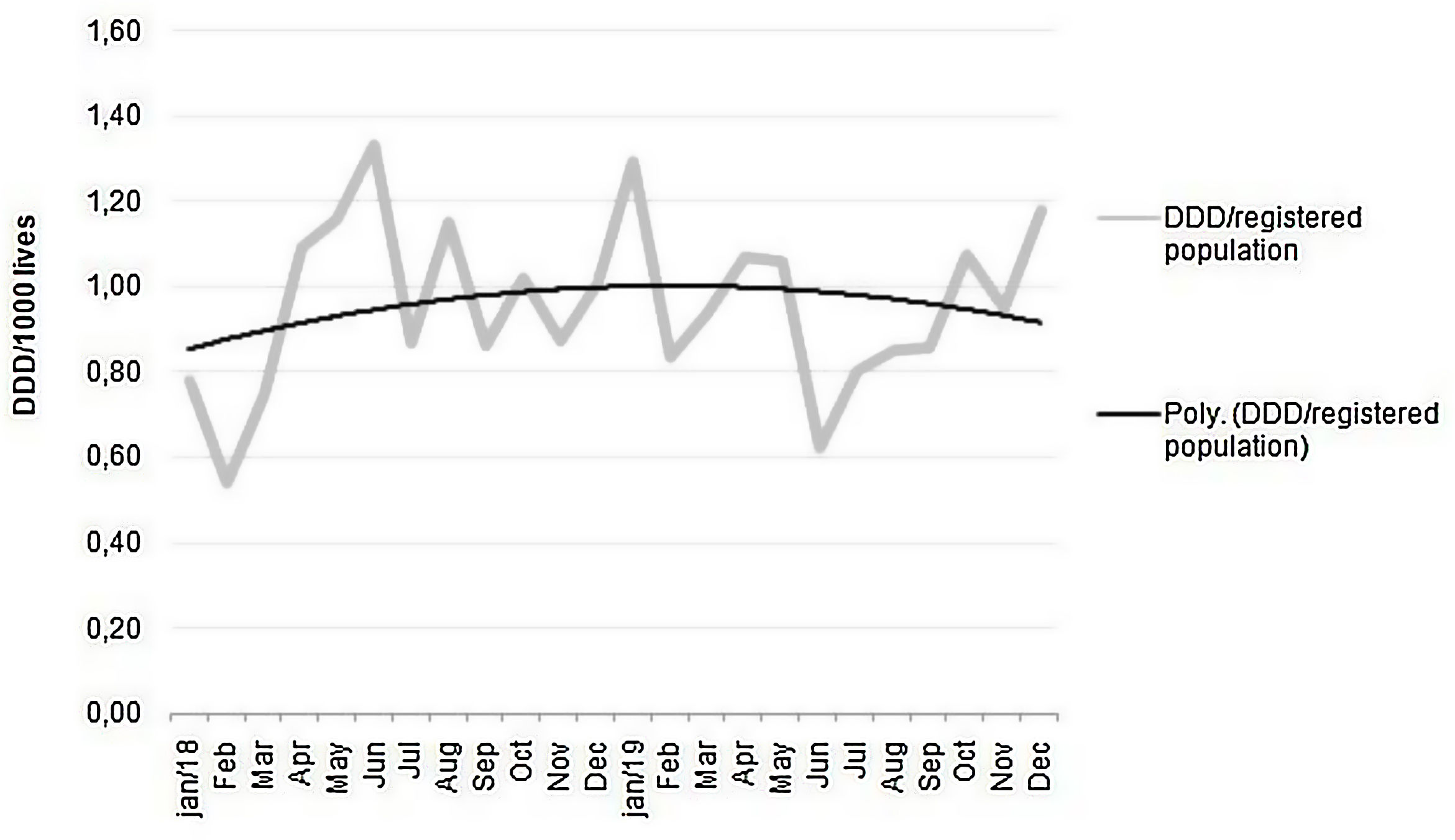
The studied population and the recommendations made on the antimicrobial requirements were characterized by means and percentiles, as well as the consumption of main antimicrobials before and after the intervention. We also calculated the monthly global consumption of antimicrobials in DDD for every 1000 members of the HMO, and an order 2 polynomial trend line was used to assess the impact of the program.
This study was approved by Invitare Research Ethics Committee, to which the HMO is linked, complying with the ethical rules of regulation in Brazilian research, according to Resolution 466 of December 12, 2012, of the National Health Council and Ministry of Health.
ResultsFrom February to December 2019, 506 antimicrobial requests were evaluated. Only 27 patients were referred for face-to-face evaluation by ID physician before defining antibiotic therapy.
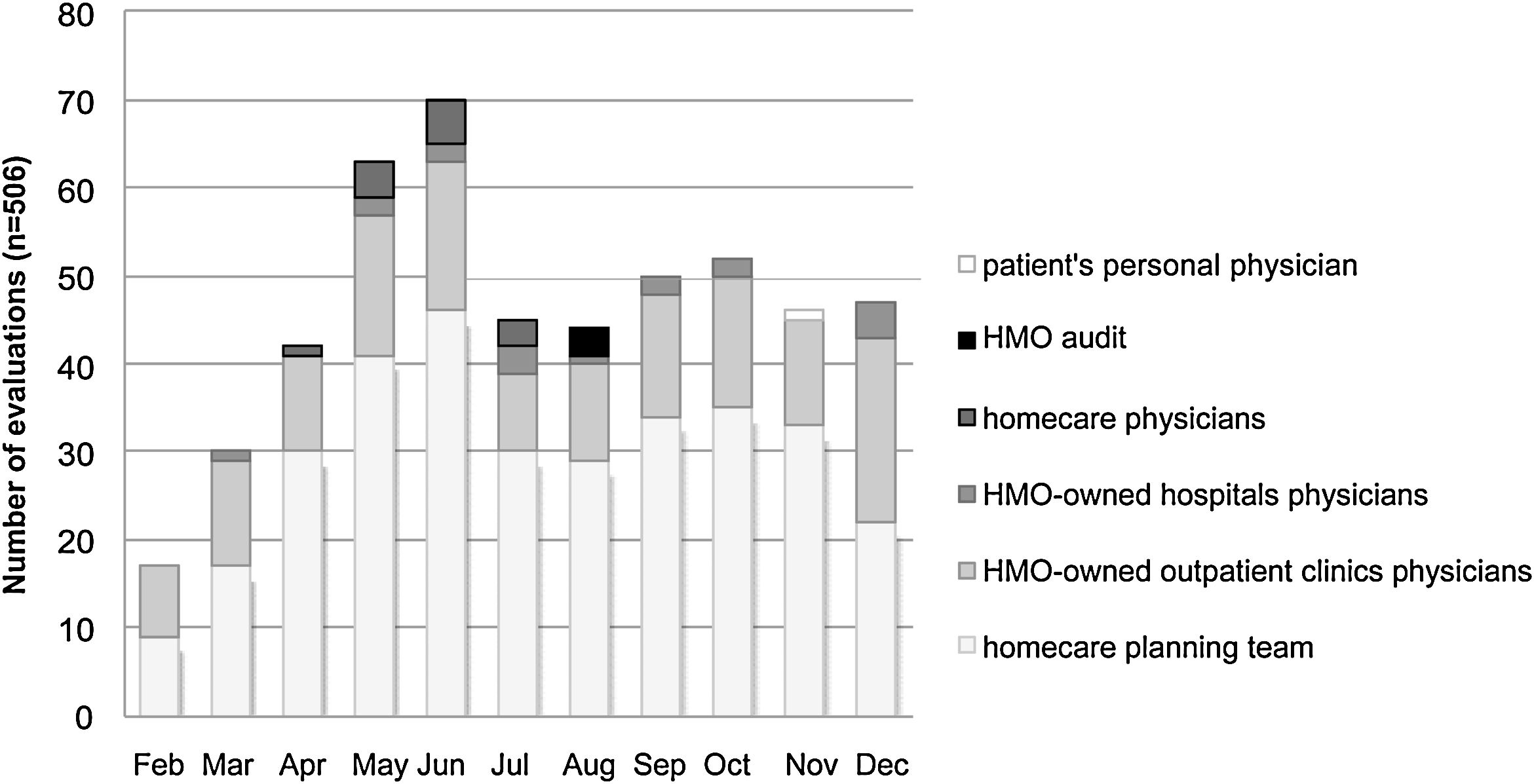
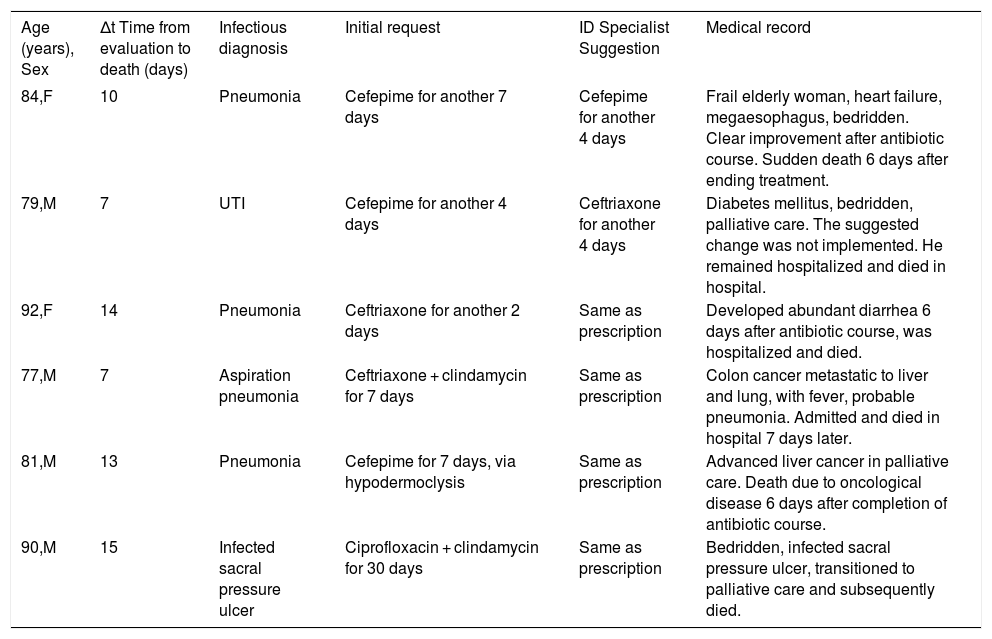
The number of evaluations increased between February and June (Fig. 1) as a result of widespread internal communication of the ID specialist availability, in addition to the progressive increase in the HMO's membership during this period. In July, there was a reduction in the number of assessments due to the creation of a separate antimicrobial management program for the largest hospital in the HMO. The number of assessments remained stable until December (Fig. 1).
The healthcare professionals who requested the greatest number of evaluations were the nursing teams responsible for planning homecare services after hospital discharge (Fig. 1). The HMO-owned outpatient clinic medical team was the second most demanding group of the consulting service. Among the HMO-owned outpatient clinics and hospitals, it is worth noting that some physicians started a partnership with our ID specialist, and over time changed their own antimicrobial prescribing patterns.
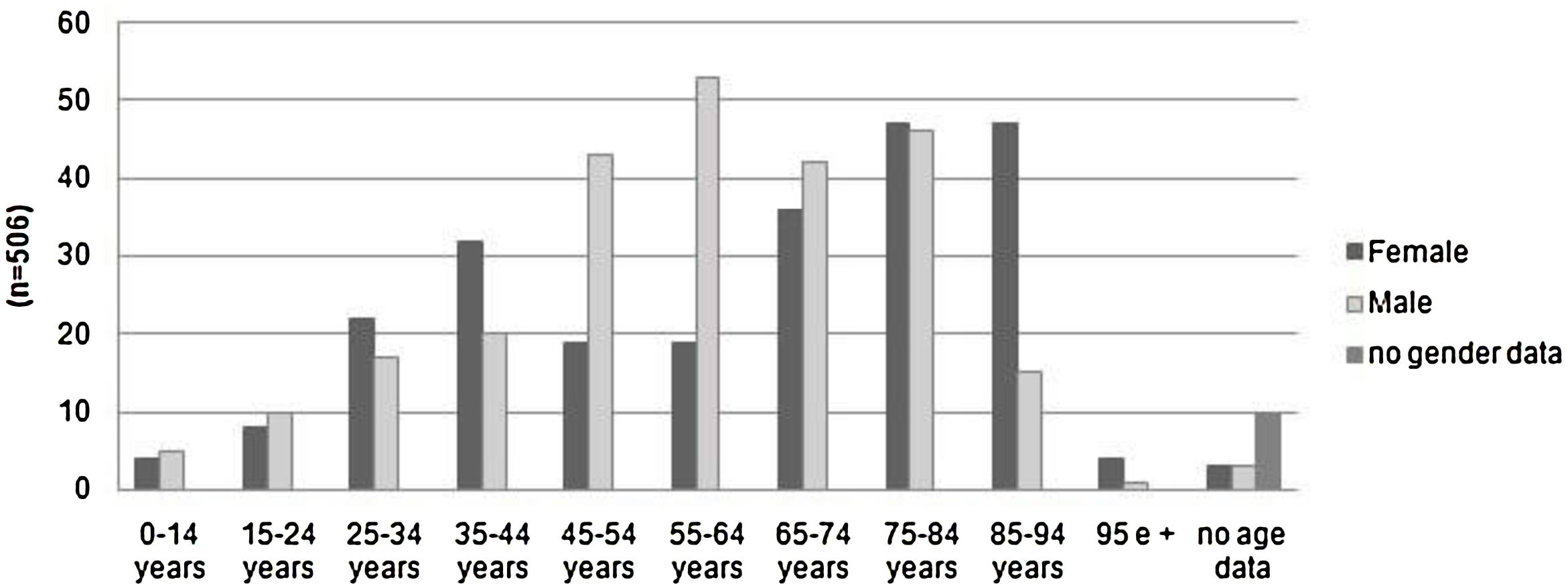
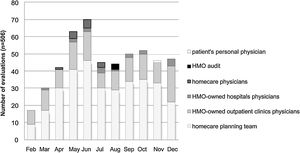
Patients over 65 years old represented 49% of the evaluations. Only 2% were pediatric cases. The number of assessments rose progressively with increasing age, dropping off only after age 85. Among patients 45–64 years old, there was a clear male predominance. Women represented a greater number of cases over 85 years old (Fig. 2).
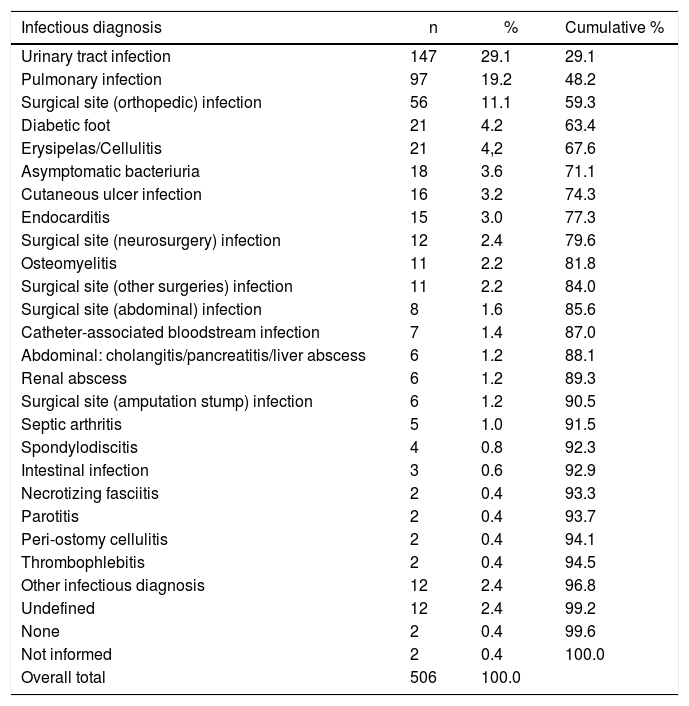
The most frequent diagnoses were urinary tract infection, pneumonia, and orthopedic surgical site infection, which together accounted for 59% of all cases (Table 1).
Infectious diagnoses associated with OPAT evaluations, February to December 2019.
| Infectious diagnosis | n | % | Cumulative % |
|---|---|---|---|
| Urinary tract infection | 147 | 29.1 | 29.1 |
| Pulmonary infection | 97 | 19.2 | 48.2 |
| Surgical site (orthopedic) infection | 56 | 11.1 | 59.3 |
| Diabetic foot | 21 | 4.2 | 63.4 |
| Erysipelas/Cellulitis | 21 | 4,2 | 67.6 |
| Asymptomatic bacteriuria | 18 | 3.6 | 71.1 |
| Cutaneous ulcer infection | 16 | 3.2 | 74.3 |
| Endocarditis | 15 | 3.0 | 77.3 |
| Surgical site (neurosurgery) infection | 12 | 2.4 | 79.6 |
| Osteomyelitis | 11 | 2.2 | 81.8 |
| Surgical site (other surgeries) infection | 11 | 2.2 | 84.0 |
| Surgical site (abdominal) infection | 8 | 1.6 | 85.6 |
| Catheter-associated bloodstream infection | 7 | 1.4 | 87.0 |
| Abdominal: cholangitis/pancreatitis/liver abscess | 6 | 1.2 | 88.1 |
| Renal abscess | 6 | 1.2 | 89.3 |
| Surgical site (amputation stump) infection | 6 | 1.2 | 90.5 |
| Septic arthritis | 5 | 1.0 | 91.5 |
| Spondylodiscitis | 4 | 0.8 | 92.3 |
| Intestinal infection | 3 | 0.6 | 92.9 |
| Necrotizing fasciitis | 2 | 0.4 | 93.3 |
| Parotitis | 2 | 0.4 | 93.7 |
| Peri-ostomy cellulitis | 2 | 0.4 | 94.1 |
| Thrombophlebitis | 2 | 0.4 | 94.5 |
| Other infectious diagnosis | 12 | 2.4 | 96.8 |
| Undefined | 12 | 2.4 | 99.2 |
| None | 2 | 0.4 | 99.6 |
| Not informed | 2 | 0.4 | 100.0 |
| Overall total | 506 | 100.0 |
There was large variability in the potential severity of cases, as seen in the diagnoses detailed in Table 1.
Of the requested evaluations, 6% (30/506) were not completed due to insufficient information, and 12% (60/506) corresponded to requests for antimicrobial guidance by the prescribing physician, without a previously defined treatment plan.
Thus, 416 evaluations of prescriptions with a defined treatment plan were completed. Antimicrobial prescriptions were approved with no suggested changes in 42% of cases (176/416). In 58% (240/416) of evaluations, modifications were recommended to the primary physician.
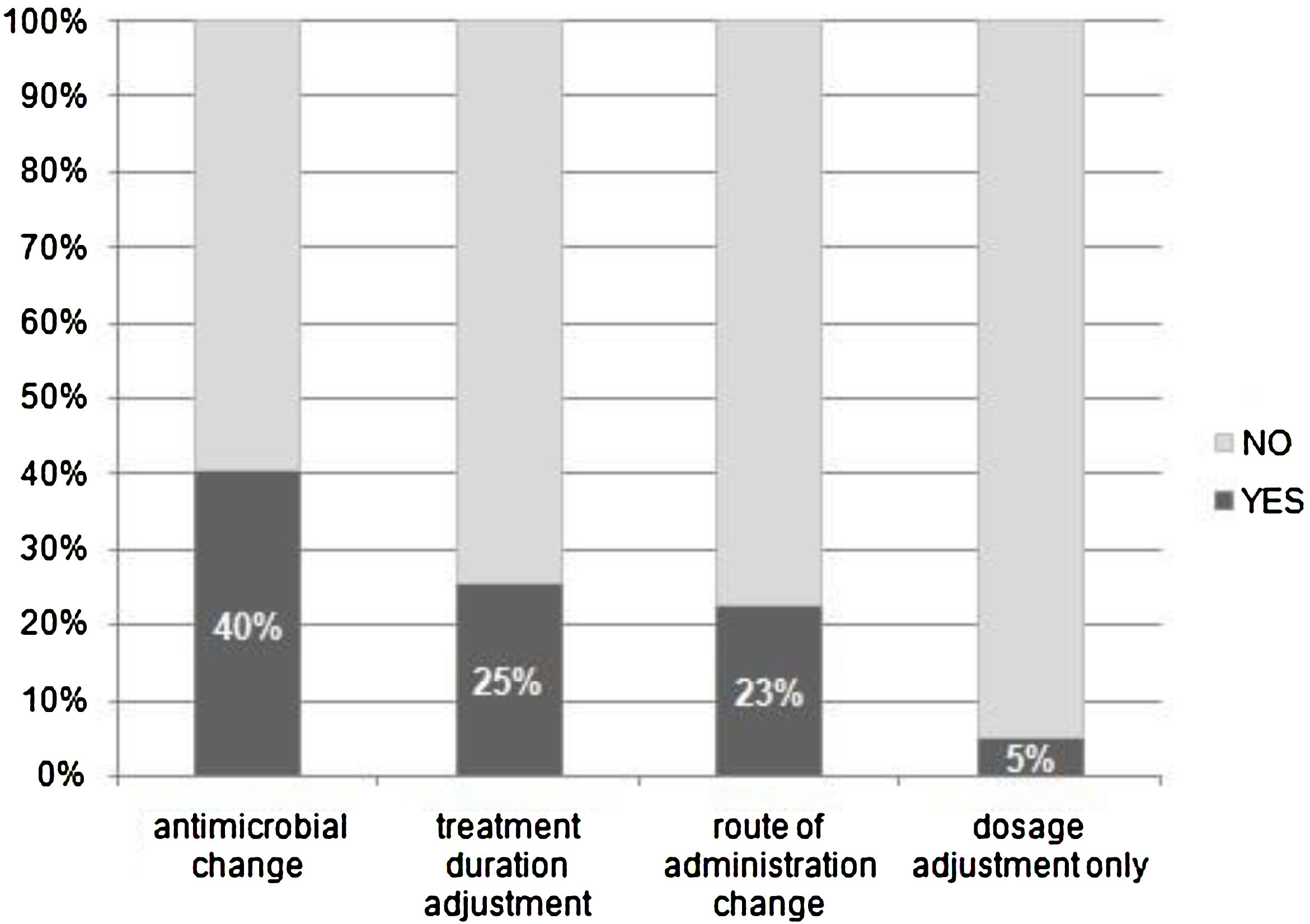
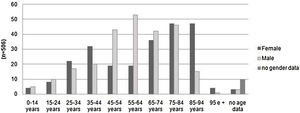
Of the 416 evaluations, the most frequent recommendations were changes in antimicrobial agent (40%), treatment duration (25%), route of administration (23%), and dose adjustment (5%) (Fig. 3).
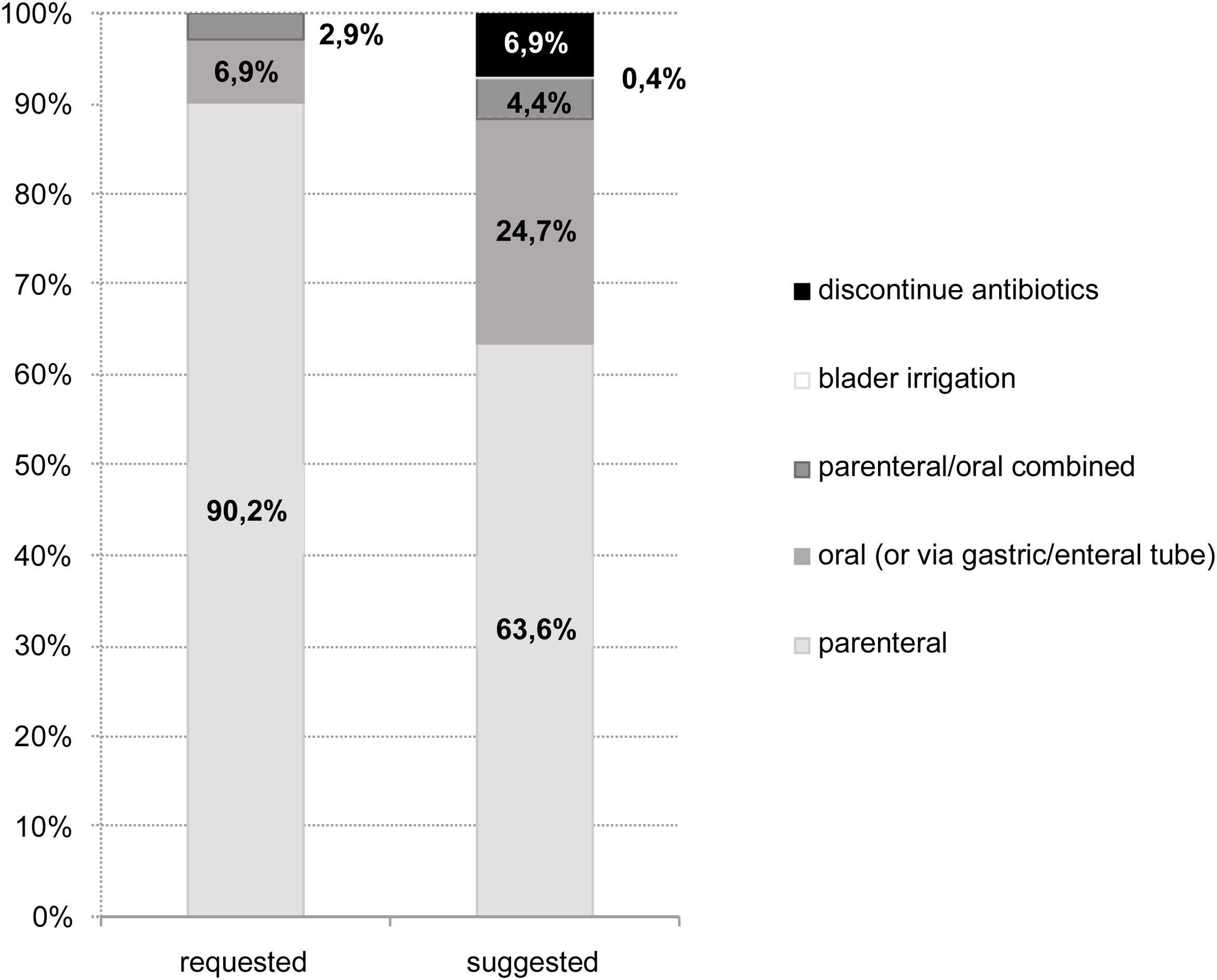
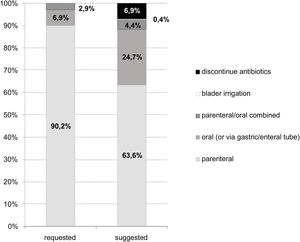
Compared to the initial prescriptions, the specialist-recommended antimicrobial regimens had a higher proportion of oral therapies and a lower proportion of intramuscular or intravenous medications (Fig. 4). There was also a significant proportion (7%) of evaluations that recommended discontinuation of antimicrobial treatment, either because the treatment period had already been completed or because there was no evidence of infection. Bladder irrigation with amphotericin was indicated in two fungal infections.
The average duration of treatment was shorter in the suggested treatment courses (13.3 days), compared to the initial requested therapy plans (16.9 days). However, in both cases, the median duration of treatment was 7 days. Of note, among the cases in which cultures identified an infectious agent, 23% of prescriptions were considered inadequate and required guidance.
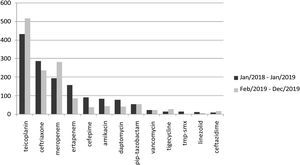
Analysis of home care pharmacy data indicated a change in antimicrobial consumption pattern after initiation of the stewardship program in February 2019 (Fig. 5). There was an increase in the use of teicoplanin and meropenem, and a decrease in ceftriaxone, ertapenem, cefepime, amikacin, and daptomycin use.
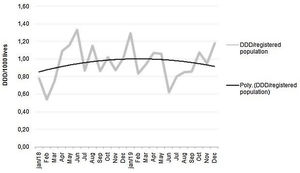
The monthly parenteral antimicrobial dispensing rate showed an upward trend in 2018, which was reversed to a downwards trend after introduction of the stewardship program (Fig. 6).
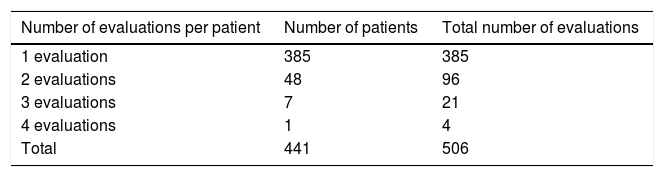
The 56 patients who required two or more consultations (Table 2) were reviewed, in order to verify whether subsequent consultations indicated any treatment failure by the ID recommendations. Two cases were detected in which the suggested changes may have resulted in treatment failure. Subsequently, these two patients underwent new treatments, both with favorable outcomes.
In addition, three cases of lower urinary tract infections required intravenous treatment based on culture results, after failure of prior empirical oral treatment. However, these infections had low morbidity risk, so the providers felt that an initial attempt of empirical oral therapy was justified, and closely monitored these patients for treatment failure and the need to adjust the antimicrobial regimen.
Six deaths occurred within 15 days of antimicrobial evaluation. All six patients had chronic illnesses and were in palliative care, and the final events leading to death showed no association with changes in antimicrobial treatment proposed by the ID consultation program (Box 1).
Deaths occurring within 15 days after the antimicrobial evaluation, 2019.
| Age (years), Sex | Δt Time from evaluation to death (days) | Infectious diagnosis | Initial request | ID Specialist Suggestion | Medical record |
|---|---|---|---|---|---|
| 84,F | 10 | Pneumonia | Cefepime for another 7 days | Cefepime for another 4 days | Frail elderly woman, heart failure, megaesophagus, bedridden. Clear improvement after antibiotic course. Sudden death 6 days after ending treatment. |
| 79,M | 7 | UTI | Cefepime for another 4 days | Ceftriaxone for another 4 days | Diabetes mellitus, bedridden, palliative care. The suggested change was not implemented. He remained hospitalized and died in hospital. |
| 92,F | 14 | Pneumonia | Ceftriaxone for another 2 days | Same as prescription | Developed abundant diarrhea 6 days after antibiotic course, was hospitalized and died. |
| 77,M | 7 | Aspiration pneumonia | Ceftriaxone + clindamycin for 7 days | Same as prescription | Colon cancer metastatic to liver and lung, with fever, probable pneumonia. Admitted and died in hospital 7 days later. |
| 81,M | 13 | Pneumonia | Cefepime for 7 days, via hypodermoclysis | Same as prescription | Advanced liver cancer in palliative care. Death due to oncological disease 6 days after completion of antibiotic course. |
| 90,M | 15 | Infected sacral pressure ulcer | Ciprofloxacin + clindamycin for 30 days | Same as prescription | Bedridden, infected sacral pressure ulcer, transitioned to palliative care and subsequently died. |
F, female; M, male; UTI, urinary tract infection.
Over the past several years, there has been a substantial increase in OPAT and a resulting need to develop management strategies to ensure its proper use and avoid associated excessive costs and risks.3,4 Accordingly, our HMO’s administrative data from 2018 showed a progressive increase in the use of intravenous antimicrobials in home care treatments, prompting an in-depth assessment of OPAT use and suitability within our organization. In February 2019, we decided to implement a remote antimicrobial stewardship program in our HMO, and an ID physician was appointed to conduct this assessment. Available remotely full- time, this specialist conducted a consultation program that did not involve extra technological resources, as it was carried out through the usual means of communication. Our program had a wide reach within the HMO network and was able to modify the pattern of antimicrobial use. This initiative resulted in cost reduction for the HMO, with no detrimental effects observed in the quality of patient care.
A detailed record of the remote ID evaluations was key in determining the positive results of this intervention. Confirming findings from previous studies,4 we observed opportunities for intervention. These included replacing intravenous antibiotic treatment with oral therapy (thus avoiding the risks associated with venous access), as well as making antimicrobial adjustments based on identification of the etiologic agent in cultures. These interventions corresponded to the most appropriate use of antimicrobials and benefited patients’ clinical outcome, although this effect had not been measured in the present study.
Since this study was not previously planned, but resulted from the observation of a dynamic assistance situation, it was not possible to carry out a more robust statistical analysis. The method for assessing antimicrobial requests was not put in place suddenly as in a planned study. Rather, it was progressively implemented over the months. During the year of initiation of the antimicrobial stewardship program, the HMO incorporated other companies and there were changes in its membership composition, not only due to increase in the number but also in the age composition, which became older. Incorporation of older members led to an increase of antimicrobials consumption of patients on home care. However, availability of ID specialist consultation reversed the trend for greater consumption, which can be taken as indicative of a success of the initiative, although more controlled studies ought to be carried out to confirm this finding.
There was a low rate of treatment failure or associated death in the 506 evaluations performed. Two cases (0.4%) of treatment failure, possibly resulting from the ID specialist’s recommendations were identified. These treatments were subsequently modified, with favorable outcomes in both cases. However, recognition of these therapeutic flaws draws attention to the need for rigorous intervention criteria, as well as limiting these recommendations depending on the availability of reliable remote information. In this study, no deaths were identified as associated with the treatment changes recommended by the ID physician.
Receptivity to the remote stewardship program varied among the prescribing professionals. Some physicians became systematic users of the program and noticeably changed their own pattern of antimicrobial prescription. Others resisted to implement the ID specialist’s suggestions, particularly those who were part of the HMO’s external network. Treatment guidelines for antibiotics of high oral bioavailability were frequently questioned by the prescribing physicians, although their effectiveness, similar to that of the intravenous therapy, has been described for over two decades and in different clinical scenarios.7,8
Qualitative studies have indicated that multiple factors interfere with the adherence of prescribing physicians to antimicrobial stewardship programs.9,10 Interventions that interrupt treatments already started are not well accepted. On the other hand, the opportunity to consult a guideline or to promptly discuss the case with the specialist are educational measures to engage the prescriber in the antimicrobial stewardship. Presence of the ID specialist as a facilitator is usually more effective than restrictive measures.
The stewardship program was used a consultation management model, with critical assessment but no prohibition of the prescriber's initial antimicrobial plan, and the analysis of the home care pharmacy data revealed an impact on subsequent pattern of intravenous antimicrobial use. Resistance presented by some prescribers towards the program, including providing insufficient information to complete the evaluations, indicates the need to promote the standardization of this approach,11,12 so it can be accepted to a greater degree by the medical community.
In our study, the greatest number of antimicrobial evaluations were requested by the nursing teams responsible for planning home care services after hospital discharge. There were no participation of clinical pharmacists, and we consider that the process would have been more effective with the participation of these professionals with specific knowledge in this matter.
Part of the patients who received home care services did not have mobility restrictions. Therefore, the use of infusion centers is promising towards increasing procedure safety and rationalization of resources. While not yet widely available in developing countries such as Brazil, it is already more readily accessible in some countries, which have been delivering OPAT in infusion centers.13
The results of our study indicate that an outpatient stewardship program, in the form of remote consultations provided by an ID physician, proved to be effective and safe in the home care setting. Institutional standardization of this program is necessary to increase its acceptance by healthcare services and professionals.
Funding statementWe acknowledge the NotreDame Intermédica Research Institute for the financial support.
Conflicts of interestThe authors declare no conflicts of interest.
We acknowledge the great contribution of Ana Cláudia Onuchic-Whitford, who reviewed the scientific language of the text in English.