Some COVID-19 patients have higher mortality and the responsible factors for this unfavorable outcome is still not well understood.
ObjectiveTo study the association between ferritin levels at admission, representing an inflammatory state, and hospital mortality in COVID-19 patients.
MethodsFrom May through July 2020, SARS-CoV-2 positive patients with moderate to severe clinical symptoms were evaluated at admission, regarding clinical and laboratory data on renal and hepatic function, hematologic parameters, cytomegalovirus co-infection, and acute phase proteins.
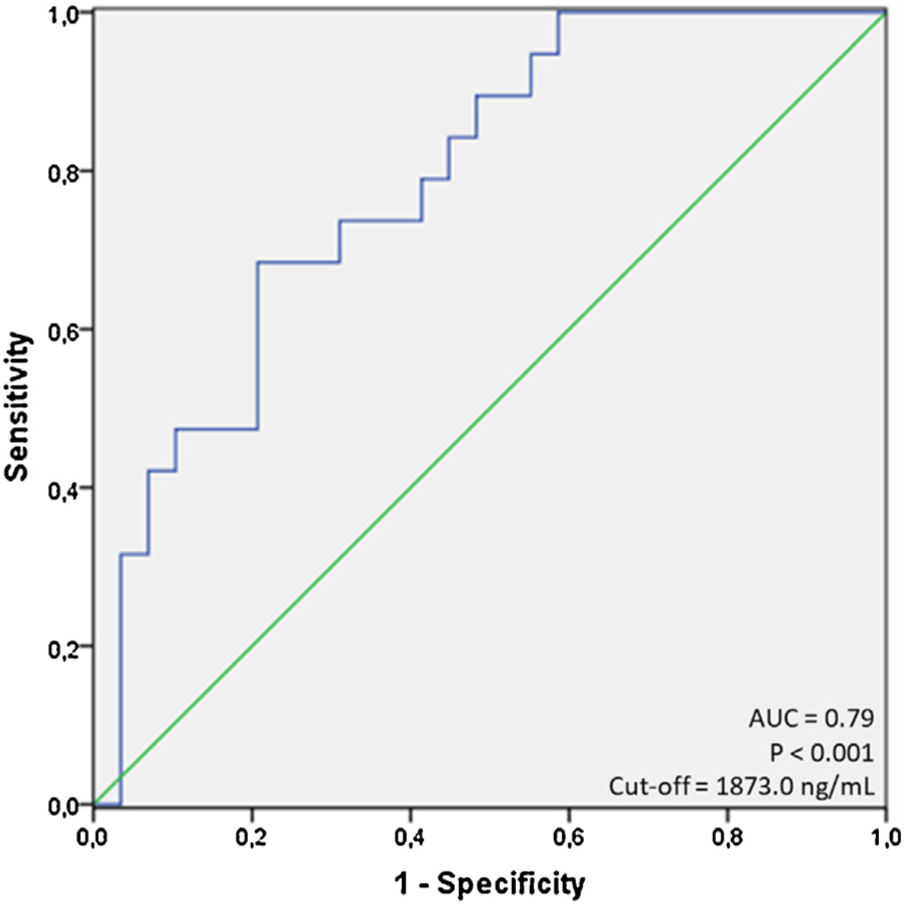
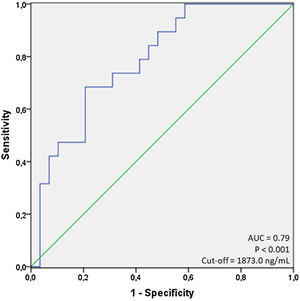
ResultsA total of 97 patients were included; mean age=59.9±16.3 years, 58.8% male, 57.7% non-white, in-hospital mortality=45.4%. Age, ferritin, C-reactive protein, serum albumin and creatinine were significantly associated with mortality. Ferritin showed area under the curve (AUC) of 0.79 (p<0.001) for the cut-off of 1873.0ng/mL, sensitivity of 68.4% and specificity of 79.3% in predicting in-hospital mortality. Age ≥60 years had an odds ratio (OR) of 10.5 (95% CI=1.8–59.5; p=0.008) and ferritin ≥1873.0ng/mL had an OR of 6.0 (95% CI=1.4–26.2; p=0.016), both independently associated with mortality based on logistic regression analysis.
ConclusionThe magnitude of inflammation present at admission of COVID-19 patients, represented by high ferritin levels, is independently predictive of in-hospital mortality.
It is known that some COVID-19 patients have poor outcomes and the factors responsible for determining these unfavorable evolution are still unsuccessfully understood. However, age and some comorbidities such as diabetes and hypertension were the first conditions thought to be risk factors for severe COVID-19.1
Since SARS CoV-2 is a novel virus causing infection in humans, there is substantial uncertainty about how early clinical or laboratory findings could be related to infectivity and disease severity, besides how these parameters could contribute to increase mortality. In this context, studies have shown that the exacerbated inflammatory response (cytokine storm) can directly impair organ function in COVID-19 patients with moderate to severe disease, leading to decompensation, organ dysfunction and death.2
In this report, we aimed to study the relationship between serum ferritin levels, measured at the moment of hospitalization, and general mortality among COVID-19 patients admitted to a high complexity university hospital. In addition, we also investigated if there is a ferritin cut-off value that could be predictive of death as the final outcome, which could be very useful in clinical practice for following up moderate to severe COVID-19 cases.
Material and methodsStudy designWe retrospectively studied patients diagnosed with SARS-CoV-2 infection during the period of May through July 2020. This study focused on the analysis of common determinants and laboratory results obtained in the first days following hospital admission which could be associated with clinical outcomes (survival or death). In addition, cytomegalovirus (CMV) viral load was investigated since immunocompromised patients receives care at our hospital, where CMV infection could be an important cause of morbidity and mortality. This study was approved by the Ethics Committee of the Universidade Federal Fluminense (CAAE: 30623520.5.0000.5243).
Patients and data collectionPatients included in this study were admitted to the Hospital Universitario Antônio Pedro (HUAP – Niterói, Rio de Janeiro, Brazil) during the initial phase of COVID-19 pandemic in Brazil. HUAP is a reference hospital for the Metropolitan Region II of Rio de Janeiro State, which encompasses seven municipalities (about two million inhabitants). So, HUAP is the reference center for high complexity cases in this region, which includes cancer, autoimmune disease, heart surgeries, and transplants; being also the current reference treatment center for moderate to severe COVID-19 cases (e.g. persistent cough, fever and respiratory discomfort or drop in oxygen saturation). Included patients had detected RT-PCR for SARS-CoV-2 within the first week from the onset of symptoms. Patient data (e.g. sex, ethnicity, age, and comorbidities such as cancer, diabetes, immunosuppressive disorders, cardiovascular and chronic kidney diseases) were retrieved from medical charts.
Diagnosis of SARS-CoV-2 infectionDuring the COVID-19 pandemic, the Multiuser Laboratory for Research Support in Nephrology and Medical Science (LAMAP) located at HUAP has been equipped to perform RNA extraction and RT-PCR to diagnose SARS-CoV-2 infection, operating in accordance with the Brazilian Ministry of Health regulations. Briefly, viral RNA was isolated from nasopharyngeal swabs or tracheal aspirates from subjects with suspected COVID-19 using the QIAamp Viral RNA kit (Catalog no. 52906, QIAGEN, Hilden, Germany) according to the manufacturer's instructions. Subsequently, using the 2019-nCOV RUO Kit (Catalog no. 10006770, Integrated DNA Technologies, Inc – IDT, Iowa, USA) and GoTaq® Probe 1-Step RT-qPCR (Catalog no. A6121, Promega Corporation, Wisconsin, USA) the assay was performed in three separate reactions per specimen for each target (N1, N2, and the internal control RNAaseP). Finally, amplification was performed using the 7500 System (Applied Biosystems, ThermoFisher Scientific, California, USA).
Laboratory testsBiochemical and hematological parameters were assessed by automated methods using Siemens Dimension RxL MaxR (Siemens, Newark, Delaware, USA), Coulter LH 750R (Beckman Coulter, California, USA) and Sysmex CA-1500 SystemR (Sysmex America Inc., Illinois, USA) equipments. International normalized ratio (INR) was calculated. Leukopenia was defined as a value lower than 4000leukocytes/mm3 and lymphopenia as a value lower than 1000lymphocytes/mm3. All the routine tests were performed at the Clinical Pathology Service (HUAP/UFF).
Statistical analysisData are expressed as mean±standard deviation (SD) or n (%). We divided the cases into two groups at the end of hospitalization time: patients who remained alive and patients who had died. For continuous variables, differences between two groups or more were assessed by t test or Mann–Whitney test and ANOVA or Kruskal–Wallis test with the correspondent post-tests, according to the variable distribution. Two-sided chi-square test was used to compare differences between proportions of categorical variables. The ability of a test to predict the primary clinical outcome or status (hospital discharge vs. death) was evaluated by the area under curve (AUC) after performing a receiver operating characteristic curve (ROC). Subsequently, using cut-offs for a variable, we performed the calculation of positive and negative predictive values. For the stepwise logistic regression, all variables presenting p-value ≤0.1 in uni-variate analysis were included in the initial binary model considering outcome (survival or death) as the dependent variable to estimate odds ratios (OR). Data were analyzed using statistical package (SPSS) and p-values were considered significant when <0.05.
ResultsDuring the months of May through July 2020, we received respiratory tract samples of hospitalized patients to assess COVID-19 suspected cases. A total of 97 SARS-CoV-2 positive patients were diagnosed. The overall mean age (±SD) was 59.9±16.3 years, 57/97 (58.8%) were male; 42.3% self-declared to be white, 9.3% black, 23.7% brown, 1.0% Asian, and that was no information for 23.7%. According to clinical severity, patients were admitted at the emergence room, infectious disease unit or intensive care unit. General symptoms presented at the time of hospital admission were mainly fever, cough, sore throat, sneezing, loss of taste, and eventually diarrhea or abdominal pain. In general, prostration and prominent dyspnea with O2 saturation <95% associated to a ground glass pattern on thoracic tomography (>50%) were also frequently observed (cases were moderate to severe), constituting a typical presentation of hospitalized cases in our center. Overall, 44 (45.4%) patients died due to COVID-19. Biochemistry tests were performed within the first 24–48h of hospitalization. We carefully checked the exact day of respiratory samples collection for SARS-CoV-2 detection and the day of symptoms onset. In our institution we have a CMV screening routine for laboratory monitoring in immunosuppressive patients and these results were also included, but with no statistical significance.
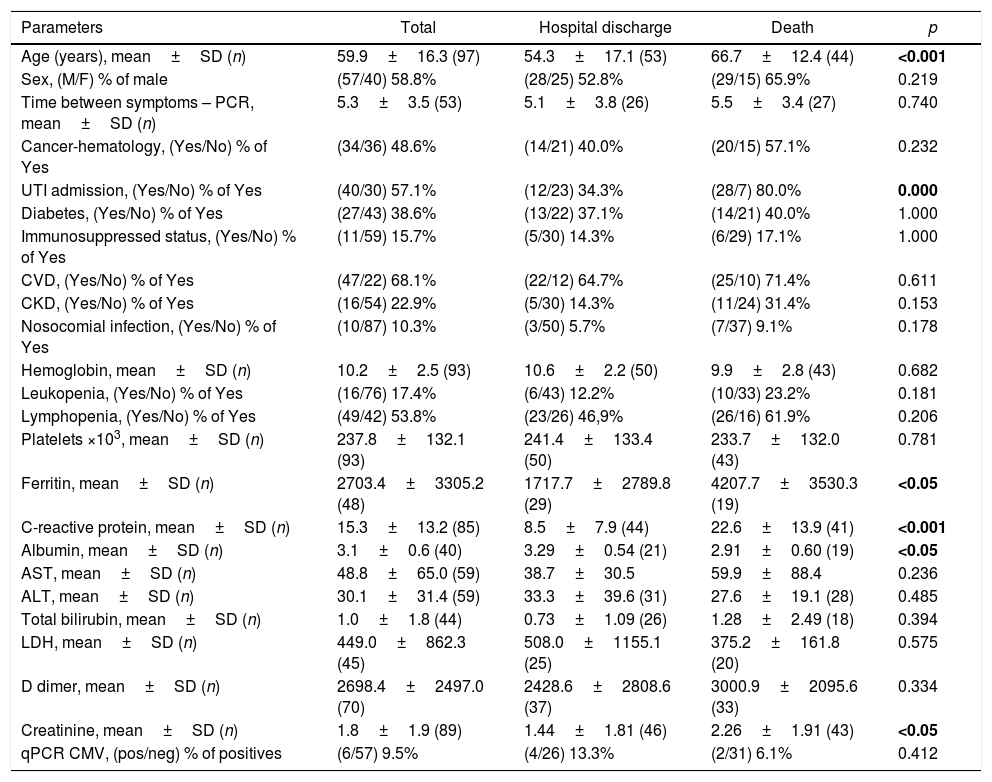
As shown in Table 1, the first part of the evaluation was a univariate analysis comparing the two groups (survival vs. death). Overall, there were statistical differences in age (p<0.001), serum levels of ferritin (p<0.05), C-reactive protein (p<0.001), albumin (p<0.05), and creatinine (p<0.05). In addition, admission to intensive care unit (ICU) was also significantly associated (p<0.0001). Importantly, serum ferritin levels at admission were not statically different between patients with and without immunosuppressive conditions (2661±3188ng/mL vs. 3028±3644ng/mL, respectively; p=0.9).
Demographic and laboratory characteristics of 97 hospitalized SARS-CoV-2 positive patients according to survival status at the end of the study.
| Parameters | Total | Hospital discharge | Death | p |
|---|---|---|---|---|
| Age (years), mean±SD (n) | 59.9±16.3 (97) | 54.3±17.1 (53) | 66.7±12.4 (44) | <0.001 |
| Sex, (M/F) % of male | (57/40) 58.8% | (28/25) 52.8% | (29/15) 65.9% | 0.219 |
| Time between symptoms – PCR, mean±SD (n) | 5.3±3.5 (53) | 5.1±3.8 (26) | 5.5±3.4 (27) | 0.740 |
| Cancer-hematology, (Yes/No) % of Yes | (34/36) 48.6% | (14/21) 40.0% | (20/15) 57.1% | 0.232 |
| UTI admission, (Yes/No) % of Yes | (40/30) 57.1% | (12/23) 34.3% | (28/7) 80.0% | 0.000 |
| Diabetes, (Yes/No) % of Yes | (27/43) 38.6% | (13/22) 37.1% | (14/21) 40.0% | 1.000 |
| Immunosuppressed status, (Yes/No) % of Yes | (11/59) 15.7% | (5/30) 14.3% | (6/29) 17.1% | 1.000 |
| CVD, (Yes/No) % of Yes | (47/22) 68.1% | (22/12) 64.7% | (25/10) 71.4% | 0.611 |
| CKD, (Yes/No) % of Yes | (16/54) 22.9% | (5/30) 14.3% | (11/24) 31.4% | 0.153 |
| Nosocomial infection, (Yes/No) % of Yes | (10/87) 10.3% | (3/50) 5.7% | (7/37) 9.1% | 0.178 |
| Hemoglobin, mean±SD (n) | 10.2±2.5 (93) | 10.6±2.2 (50) | 9.9±2.8 (43) | 0.682 |
| Leukopenia, (Yes/No) % of Yes | (16/76) 17.4% | (6/43) 12.2% | (10/33) 23.2% | 0.181 |
| Lymphopenia, (Yes/No) % of Yes | (49/42) 53.8% | (23/26) 46,9% | (26/16) 61.9% | 0.206 |
| Platelets ×103, mean±SD (n) | 237.8±132.1 (93) | 241.4±133.4 (50) | 233.7±132.0 (43) | 0.781 |
| Ferritin, mean±SD (n) | 2703.4±3305.2 (48) | 1717.7±2789.8 (29) | 4207.7±3530.3 (19) | <0.05 |
| C-reactive protein, mean±SD (n) | 15.3±13.2 (85) | 8.5±7.9 (44) | 22.6±13.9 (41) | <0.001 |
| Albumin, mean±SD (n) | 3.1±0.6 (40) | 3.29±0.54 (21) | 2.91±0.60 (19) | <0.05 |
| AST, mean±SD (n) | 48.8±65.0 (59) | 38.7±30.5 | 59.9±88.4 | 0.236 |
| ALT, mean±SD (n) | 30.1±31.4 (59) | 33.3±39.6 (31) | 27.6±19.1 (28) | 0.485 |
| Total bilirubin, mean±SD (n) | 1.0±1.8 (44) | 0.73±1.09 (26) | 1.28±2.49 (18) | 0.394 |
| LDH, mean±SD (n) | 449.0±862.3 (45) | 508.0±1155.1 (25) | 375.2±161.8 (20) | 0.575 |
| D dimer, mean±SD (n) | 2698.4±2497.0 (70) | 2428.6±2808.6 (37) | 3000.9±2095.6 (33) | 0.334 |
| Creatinine, mean±SD (n) | 1.8±1.9 (89) | 1.44±1.81 (46) | 2.26±1.91 (43) | <0.05 |
| qPCR CMV, (pos/neg) % of positives | (6/57) 9.5% | (4/26) 13.3% | (2/31) 6.1% | 0.412 |
For continuous variables, we used two tailed Mann–Whitney tests. For categorical variables, the two-sided Chi-square tests, and we show the exact number of events (yes/no) by parameter in each line.
Abbreviations: n, number; SD, standard deviation; M, male; F, female; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDH, lactate dehydrogenase; qPCR CMV, real time polymerase chain reaction.
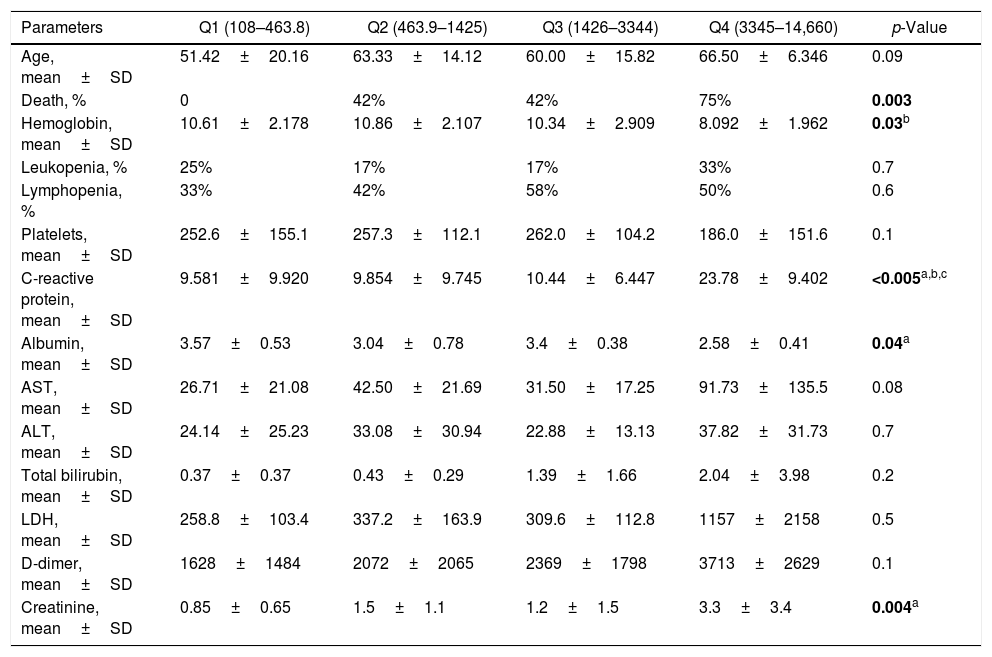
We also performed an analysis of demographic, clinical and laboratory parameters of COVID-19 hospitalized patients according to quartiles of serum ferritin obtained at admission. Patients with the highest ferritin levels (Q4 – ferritin of 3345–14,660ng/mL) also presented significant higher levels of C-reactive protein (p<0.005) and serum creatinine (p=0.04). These patients also had significantly lower levels of hemoglobin (p=0.03) and serum albumin (p=0.04). Finally, we observed an increased frequency of death by COVID-19 in the highest ferritin quartile (p=0.003), where 75% of patients in the highest quartile evolved to death. These data are presented in Table 2. Of note, we performed the analysis of other clinical parameters such as higher frequency of comorbidities according to quartiles of ferritin, but no significant differences were observed (data not shown).
Demographic and laboratory parameters of COVID-19 hospitalized patients according to ferritin quartiles.
| Parameters | Q1 (108–463.8) | Q2 (463.9–1425) | Q3 (1426–3344) | Q4 (3345–14,660) | p-Value |
|---|---|---|---|---|---|
| Age, mean±SD | 51.42±20.16 | 63.33±14.12 | 60.00±15.82 | 66.50±6.346 | 0.09 |
| Death, % | 0 | 42% | 42% | 75% | 0.003 |
| Hemoglobin, mean±SD | 10.61±2.178 | 10.86±2.107 | 10.34±2.909 | 8.092±1.962 | 0.03b |
| Leukopenia, % | 25% | 17% | 17% | 33% | 0.7 |
| Lymphopenia, % | 33% | 42% | 58% | 50% | 0.6 |
| Platelets, mean±SD | 252.6±155.1 | 257.3±112.1 | 262.0±104.2 | 186.0±151.6 | 0.1 |
| C-reactive protein, mean±SD | 9.581±9.920 | 9.854±9.745 | 10.44±6.447 | 23.78±9.402 | <0.005a,b,c |
| Albumin, mean±SD | 3.57±0.53 | 3.04±0.78 | 3.4±0.38 | 2.58±0.41 | 0.04a |
| AST, mean±SD | 26.71±21.08 | 42.50±21.69 | 31.50±17.25 | 91.73±135.5 | 0.08 |
| ALT, mean±SD | 24.14±25.23 | 33.08±30.94 | 22.88±13.13 | 37.82±31.73 | 0.7 |
| Total bilirubin, mean±SD | 0.37±0.37 | 0.43±0.29 | 1.39±1.66 | 2.04±3.98 | 0.2 |
| LDH, mean±SD | 258.8±103.4 | 337.2±163.9 | 309.6±112.8 | 1157±2158 | 0.5 |
| D-dimer, mean±SD | 1628±1484 | 2072±2065 | 2369±1798 | 3713±2629 | 0.1 |
| Creatinine, mean±SD | 0.85±0.65 | 1.5±1.1 | 1.2±1.5 | 3.3±3.4 | 0.004a |
For continuous variables, we used ANOVA or Kruskal–Wallis test with Bonferroni's or Dunn's post-tests, respectively (a, Q1 vs. Q4; b, Q2 vs. Q4; c, Q3 vs. Q4). For categorical variables, two-sided chi-square test was performed. Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDH, lactate dehydrogenase.
Next, to further test the relationship between each evaluated variable and mortality, we performed a series of ROC curves analyses as seen in Table 1. For non-significant variables (as LDH, neutrophil and lymphocytes count, for example, the ROC curves had low AUC values and a p-value less than 0.05). For the variables with a significant p-value, (as age, ferritin, C-reactive protein, serum albumin and creatinine), we identified that only age and ferritin presented a value of AUC greater than 0.7 (0.72 and 0.79; respectively) using cut-offs of 60.0 years for age, and 1873.0ng/mL for ferritin, respectively. Using this cut-off for ferritin, we observed sensitivity of 68.4% and specificity of 79.3%. Fig. 1 shows the display of ROC curve for ferritin.
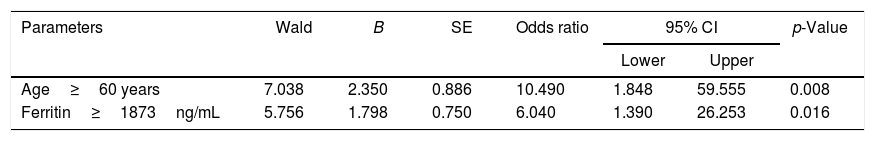
Lastly, we performed a logistic regression model for the analysis of age and serum ferritin as independent predictors of mortality. In this regard, based on a p<0.1 in univariate analysis seen in Table 1, the following variables were included in a step-wise logistic regression model: age, ferritin, C-reactive protein, serum albumin, and creatinine. In the final model, only age ≥60.0 years-old (OR: 10.5; 95% CI=1.8–59.5; p=0.008) and ferritin ≥1873.0ng/mL (OR: 6.0; 95% CI: 1.4–26.2; p=0.016) remained independently associated with mortality. Table 3 shows the final logistic regression model.
Analysis of independent predictors of mortality in COVID-19 hospitalized patients using a logistic regression model.
| Parameters | Wald | B | SE | Odds ratio | 95% CI | p-Value | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Age≥60 years | 7.038 | 2.350 | 0.886 | 10.490 | 1.848 | 59.555 | 0.008 |
| Ferritin≥1873ng/mL | 5.756 | 1.798 | 0.750 | 6.040 | 1.390 | 26.253 | 0.016 |
CI, confidence interval; B, coefficient; SE, standard error.
In December 2019, a new coronavirus was identified as responsible for cases of severe pneumonia, which was later called SARS-CoV-2.3 The virus spread quickly worldwide and subsequently, the World Health Organization (WHO) declared its associated disease, COVID-19, as an international public health emergency.4 Genome sequencing of this virus was carried out5 and led to the development of specific diagnostic tests based on reverse transcription real time-PCR (RT-qPCR).6
The acute phase reaction of an inflammatory process consists of a series of physiological and metabolic changes that begin immediately after tissue injury.7 Among the numerous systemic manifestations of this acute phase reaction is the variation in the concentrations of various plasma proteins, which are called “acute phase proteins”. Among these, the best known in clinical practice are C-reactive protein, amyloid substance serum A, haptoglobin, fibrinogen and ferritin.8 Serum ferritin has been long studied as a marker of iron metabolism,9 however, its application as biomarker of inflammation has far presented high importance in the context of COVID-19 progression, as demonstrated by previous studies in the field.10 Ferritin is an acute phase reactant, and as such, is generally elevated in inflammatory responses of any type. An initial assessment to identify the laboratory findings most commonly associated with the cytokine storm syndromes include a complete blood count, serum levels of ferritin, liver function tests, and others. These tests are readily available in the majority of health care facilities.
We studied, at the first days of hospital admission, a series of SARS-CoV-2 infected patients and we observed a strong correlation between serum ferritin and overall mortality, independently of age. A worse prognosis frequently is associated with a more rapid evolution to intensive and respiratory care or even dialysis. This is a concise laboratory-based report, which sought to answer whether we are able to identify any relevant role for risk prognosis using complementary tests routinely requested in positive SARS-CoV-2 hospitalized patients. The magnitude of the inflammation the patient already has at admission when the RT-PCR for SARS-CoV-2 is performed, seems to have a prognostic value.
There is increasing evidence that circulating ferritin levels may not only reflect an acute phase response but may play a critical role in inflammation.11 For most clinicians dealing with inflammatory diseases, serum ferritin levels are a rather non-specific marker of the acute phase response, which is often ignored or not measured when the patient is acutely ill. In some diseases, ferritin levels may be extremely high and, while not specific, these very high levels may be helpful in identifying patients at risk.12 In some previous studies, ferritin levels were more elevated in older and/or hypertensive participants, which was also associated with increased mortality.13
We emphasize that the profile of COVID-19 patients in our hospital is different from the general population, as they have advanced age, cardiovascular disease and diabetes, all correlated with higher mortality. We are a reference center for high complexity cases in our region. We highlight that 48.6% of our population had onco-hematological diseases and 22.0% had chronic kidney disease. A very relevant point in this study is the searching for a complementary routine exam that could be used as an independent predictor of mortality at the time of hospital admission.
In our study, CMV viral load at admission was not significantly correlated with final outcomes. CMV reactivation and risk for CMV disease possibly related with a subjacent immunosuppressive status may occur as in systemic lupus erythematous or in organ transplant14,15; however, characteristics of latency and reactivation of the herpes family should be better monitored in the critically ill COVID-19 patients. For example, many of the reported cases and series of CMV-associated to cytokine storm syndromes involve viral reactivation during immune suppressed states.16 The rate of CMV seroprevalence increases with age. CMV has been shown to affect peripheral T cell phenotypes, increase inflammatory mediated cytokines such as IL-6 and play a role in immune dysregulation. The role of CMV in those with severe COVID-19 disease merits exploration.17
A study focusing on early hyperinflammation in COVID-19 patients evaluated the high levels of serum ferritin in the first seven days of hospitalization as a predictor for the cytokine storm syndrome,18 and a meta-analysis including 21 studies (3377 patients and 33 laboratory parameters) also demonstrated serum ferritin as a biomarker for potential progression to critical illness.10 Nevertheless, recent studies have also demonstrated that anti-inflammatory biomarkers could also be elevated during the acute phase of COVID-19.19,20 This highlights the importance of future studies focusing on the balance between pro- and anti-inflammatory mediators and how this could impact on disease progression.
In conclusion, this study was designed to answer whether one could identify any relevant role for prognostic risk using routine complementary exams. The magnitude of the inflammation in the first days after hospitalization, represented by a hyperferritinemic syndrome, may help to identify patients at higher risk for early clinical decisions regarding the preventive measures directed at such patients.
FundingThis study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, #001), Conselho Nacional de desenvolvimento Científico e Tecnológico (CNPq), Ministério da Educação (UFF) and The Brazilian Innovation Agency (FINEP) through Ministério da Ciência, Tecnologia e Inovações – REDE VÍRUS/Laboratórios de Campanha.
Conflicts of interestThe authors declare no conflicts of interest.
We would like to thank the Service of Clinical Pathology for laboratory tests and others undergraduate students that contribute to discussion of the manuscript, as Gilmar de Souza Lacerda.