Hepatitis C virus (HCV) infection continues to be an important public health problem worldwide. Despite the availability of drugs that promote the cure of infection in more than 95% of cases, the identification of HCV carriers remains a major challenge.
ObjectiveTo evaluate a strategy for identifying HCV carriers based on combined criteria: screening in emergency units and specialty outpatient clinics of a tertiary hospital and among older adults (≥45 years), both suggested as efficient in epidemiological studies.
MethodsA cross-sectional, analytical and descriptive study was conducted on individuals of both sexes, aged 45 years and older, attending the emergency department and specialty outpatient clinics of a University Hospital in São Paulo, Brazil, from January 2016 to June 2018. After giving formal consent, the patients were submitted to a standardized interview and rapid testing for the identification of HCV antibodies (SD BIOLINE® anti-HCV).
ResultsA total of 606 adult patients (62% women and 37% men) were evaluated. The mean age was 62±10 years. Four positive tests were identified, with confirmation by conventional serology and HCV-RNA determination. Thus, the prevalence of HCV identified in the sample was 0.66%. All patients had a history of risk factors for infection.
ConclusionThe strategies of birth-cohort testing and screening in emergency medical services for the identification of HCV carries, both suggested in the literature as efficient for the diagnosis of hepatitis C, resulted in a low rate of HCV infection. These findings highlight the magnitude of the challenge of identifying asymptomatic HCV carriers in Brazil.
Hepatitis C remains an important public health problem in many regions of the world as well as in Brazil. A population-based survey conducted in the capital cities of Brazil some years ago found a prevalence of infection with hepatitis C virus (HCV) of 1.38% in the age group of 10 to 69 years. The main routes of transmission identified were intravenous drug use, blood transfusion, sexual transmission, occupational accident, and hemodialysis.1 However, according to a more recent study, the estimated prevalence of hepatitis C in Brazil would be lower, 0.7% between 15 and 69 years.2
Likewise, the global estimate of hepatitis C has changed over time. Previously, the number of carriers of the virus was estimated at 170 million people.3 In 2017, the World Health Organization (WHO) published a report in which it reevaluated the global prevalence of infection and estimated that 71 million people worldwide were carriers of HCV.4
There are several reasons for the prevalence reduction over time. Most importantly, those infected with HCV represent a cohort that became infected in the past, mainly through blood transfusions, when the virus was not yet known; these individuals aged and many died from liver-related and non-liver-related causes, reducing the number of infected people. In addition, another reason for these divergences may be related to the diagnostic method. Some previous studies reported their results based on the detection of antibodies, which is known to differ from the diagnosis obtained based on the occurrence of viremia.4
The Brazilian Ministry of Health is signatory to an international commitment established by the WHO whose goal is to significantly reduce the number of hepatitis C cases and the complications of the disease until 2030.5 However, one of the greatest challenges to reach this WHO goal is to know the real prevalence of infection and most importantly to identify viremic patients so that they can be referred for treatment.
Several strategies have been proposed towards that end, such as those based on classical parenteral risk factors, birth-cohort screening in persons with more than 45 years-old, as well as screening in medical services that provides care for a large number of more exposed individuals.6,7 The choice of the best strategy for each country has been a matter of extensive debate considering the complexity of operational actions and costs involved.
In view of the above considerations, the aim of this study was to evaluate a strategy for identifying patients with hepatitis C, combining age-based screening of individuals >45 years and patients attending emergency department and specialty outpatient clinics of a tertiary hospital. In addition, we analyzed the characteristics of the identified patients with positive HCV serology.
Patients and methodsA cross-sectional, analytical and descriptive study was conducted at the emergency department and outpatient clinics of Hospital São Paulo (HSP), Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil, between January 2016 and June 2018. The patients were recruited consecutively in the medical specialties at both facilities. The study was approved by the Ethics Committee of UNIFESP (Approval No. 1.319.450).
Quota (non-probability) sampling was performed according to medical specialty. Men and women of the predetermined age group participated in the study. Assuming a confidence level of 95% and an interval range of 1.5%, the estimated sample size was 600 patients.
The following inclusion criteria were established: both sexes, age ≥45 years, formal agreement to participate in the study by signing the informed consent form, and attending the emergency department or specialty outpatient clinics of HSP. Patients who were in unstable conditions and those who refused to participate were excluded.
A standardized interview was carried out by the lead researcher, which consisted of sociodemographic and clinical questions related to risk factors. Before the test, the patients were individually oriented about possible results and were free to ask any question. Next, the rapid HCV test was applied. Patients with positive tests were referred to the hepatitis outpatient clinic of HSP for diagnostic confirmation and follow-up.
The rapid test developed by SD BIOLINE®, distributed in Brazil by the multinational company Alere S.A., was used. The samples were collected by a single professional previously trained and certified by the “Viral Hepatitis Diagnosis” course, a continuing education program offered by the Brazilian Ministry of Health.8 The samples were collected with a retractable lancet needle by puncture of the middle finger of one of the hands after cleaning with 70% alcohol. A 10-μL blood sample was collected immediately with a capillary pipette. The samples were transferred to the well of the device, four drops of diluent were added, followed by reading obtained within 5 to 20minutes thereafter. All rapid test positive cases were confirmed by conventional serology for determination of anti-HCV antibody and quantification of viral load (quantitative HCV RNA).
Statistical analysisContinuous variables were reported as the mean, standard deviation and range and categorical variables expressed as frequencies and percentages and compared using the chi-squared test. Student t-test for independent samples was used for comparison between care units (urgency and outpatient). Normality of the data was evaluated by the Kolmogorov–Smirnov test. A p value <0.05 indicated statistical significance. The data were analyzed using the IBM SPSS Statistics v.20.0 program (IBM Corp., Armonk, NY, USA).
ResultsOut of 606 tests, 301 (49.7%) were performed at the emergency unit and 305 (50.3%) in the outpatient specialty clinics. The outpatient specialties included cardiology (15.28%), hematology (14.95%), clinical and surgical gastroenterology (13.77%), rheumatology (11.14%), plastic surgery (9.83%), vascular surgery (9.18%), pneumology (7.97%), oncology (6.55%), endocrinology (5,98%), anesthesiology (2.95%), and nephrology (2.95%).
Overall mean age was 62±10 years and at the emergency unit and outpatient clinics were 61.8±10.4 and 63.9±10.1 years, respectively. Outpatients were significantly older than those seen at the emergency unit.
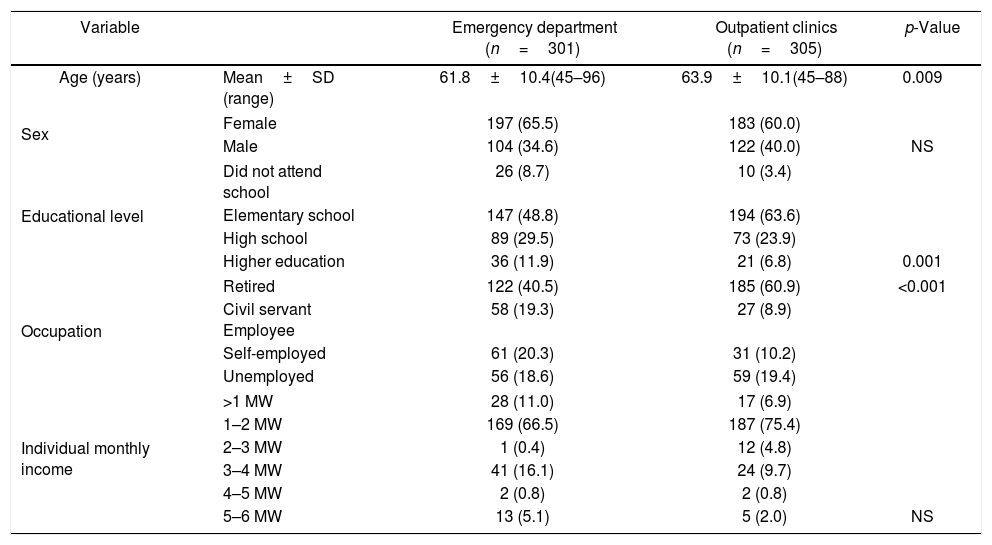
With respect to sex, 380 women (63%) and 226 men (37%) participated in the study, 104 (34.5%) men and 197 (65.5%) women seen at the emergency unit and 122 (40%) men and 183 (60%) women in the outpatient clinics. This difference was not significant. Regarding educational level, most patients had only elementary school. Outpatients had a significantly lower educational level than patients seen at the emergency unit. Retired patients were significantly more frequent in the outpatient group. There was no significant difference in income between groups. Table 1 shows the general characteristics of the sample studied.
General characteristics of the patients (n=606).
| Variable | Emergency department (n=301) | Outpatient clinics (n=305) | p-Value | |
|---|---|---|---|---|
| Age (years) | Mean±SD (range) | 61.8±10.4(45–96) | 63.9±10.1(45–88) | 0.009 |
| Sex | Female | 197 (65.5) | 183 (60.0) | |
| Male | 104 (34.6) | 122 (40.0) | NS | |
| Educational level | Did not attend school | 26 (8.7) | 10 (3.4) | |
| Elementary school | 147 (48.8) | 194 (63.6) | ||
| High school | 89 (29.5) | 73 (23.9) | ||
| Higher education | 36 (11.9) | 21 (6.8) | 0.001 | |
| Occupation | Retired | 122 (40.5) | 185 (60.9) | <0.001 |
| Civil servant Employee | 58 (19.3) | 27 (8.9) | ||
| Self-employed | 61 (20.3) | 31 (10.2) | ||
| Unemployed | 56 (18.6) | 59 (19.4) | ||
| Individual monthly income | >1 MW | 28 (11.0) | 17 (6.9) | |
| 1–2 MW | 169 (66.5) | 187 (75.4) | ||
| 2–3 MW | 1 (0.4) | 12 (4.8) | ||
| 3–4 MW | 41 (16.1) | 24 (9.7) | ||
| 4–5 MW | 2 (0.8) | 2 (0.8) | ||
| 5–6 MW | 13 (5.1) | 5 (2.0) | NS | |
SD, standard deviation; MW, minimum wage (U$ 220.00); NS, nonsignificant.
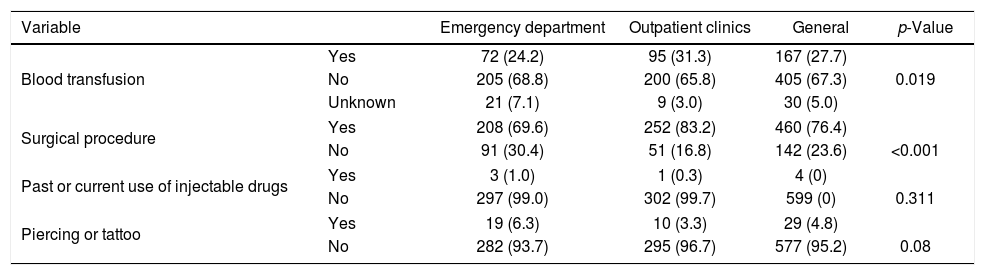
Regarding risk factors, 405 patients (67.3%) reported to have never received a blood transfusion and 30 (5%) were unaware of such procedure. Among 167 blood transfusion recipients, one (0.6%) had received a transfusion in the 1940s, six (3.6%) in the 1960s, 20 (11.7%) in the 1970s and 80s, 24 (14.4%) in the 1990s, and 96 (57.5%) after 2000. Four patients did not report the date of blood transfusion. A history of transfusion and surgical procedures were more frequent among outpatients. There were no significant differences in current or past use of injectable drugs and piercing or tattooing procedures between outpatients and emergency unit patients (Table 2).
Risk factors among the patients studied at the emergency department and specialty outpatient clinics (n=606).
| Variable | Emergency department | Outpatient clinics | General | p-Value | |
|---|---|---|---|---|---|
| Blood transfusion | Yes | 72 (24.2) | 95 (31.3) | 167 (27.7) | |
| No | 205 (68.8) | 200 (65.8) | 405 (67.3) | 0.019 | |
| Unknown | 21 (7.1) | 9 (3.0) | 30 (5.0) | ||
| Surgical procedure | Yes | 208 (69.6) | 252 (83.2) | 460 (76.4) | |
| No | 91 (30.4) | 51 (16.8) | 142 (23.6) | <0.001 | |
| Past or current use of injectable drugs | Yes | 3 (1.0) | 1 (0.3) | 4 (0) | |
| No | 297 (99.0) | 302 (99.7) | 599 (0) | 0.311 | |
| Piercing or tattoo | Yes | 19 (6.3) | 10 (3.3) | 29 (4.8) | |
| No | 282 (93.7) | 295 (96.7) | 577 (95.2) | 0.08 | |
The prevalence of anti-HCV positive among the 606 patients included in the study was 0.66% (four patients), with a 95% confidence interval of 0.18 to 1.69%. Two cases were identified at the emergency department and two cases in the outpatient clinics. All tests confirmed the presence of viremia by the HCV-RNA test, which was positive in the four cases.
Two of the four positive cases were men attending the emergency unit, aged 66 and 59 years, respectively. The other two cases were women from the outpatient clinics (pneumology and clinical oncology), both aged 70 years.
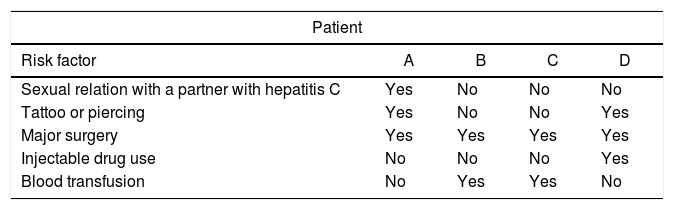
With respect to risk factors of the patients with a positive result, one patient had sexual relations with an HCV-positive partner. Two patients reported a blood transfusion and one had used intravenous illicit drugs. Regarding surgical procedures, all four patients reported to have undergone major surgery at some time during their life (Table 3).
Risk factors among patients of the sample studied with a positive hepatitis C result in the emergency department and specialty outpatient clinics.
| Patient | ||||
|---|---|---|---|---|
| Risk factor | A | B | C | D |
| Sexual relation with a partner with hepatitis C | Yes | No | No | No |
| Tattoo or piercing | Yes | No | No | Yes |
| Major surgery | Yes | Yes | Yes | Yes |
| Injectable drug use | No | No | No | Yes |
| Blood transfusion | No | Yes | Yes | No |
The prevalence of HCV infection in the sample studied, which consisted of patients older than 45 years who sought the outpatient and emergency units of a tertiary hospital, was 0.66%. This rate is in accordance with a more recent estimation based on several published and unpublished studies that indicated a prevalence of 0.7% among 15-69 years olds.2 This prevalence differs from the 1.67% reported in a Brazilian population-based study, performed 10 years ago and published in 2013, in the age group of 40 to 59 years and from the 3.41% in the age group of 60 to 69 years.1 An also higher prevalence of 1.5% was described in a population-based study conducted in the Northeastern region of Brazil in 2006.9 This reduction in the prevalence can probably be explained by the fact that many patients of older age have died since then from complications of the infection or from other causes. On the other hand, in accordance with the present study more recently published data also reported a rate of HCV infection lower than 1%.10
The same phenomenon of lower reported rates of HCV prevalence is also observed in the international literature. In the United States prior studies estimated seven million persons to be HCV-positive, a prevalence rate of 1.8%. More recent studies reported a prevalence rate of 0.9%.11 A study conducted in Egypt, the country with the highest prevalence of hepatitis C in the world, also revealed a decline in the prevalence of infection. The seroprevalence in the population was 10% in 2015 compared to 14.7% in 2008. Taken together, these findings show a clear decreasing trend in the prevalence of hepatitis C worldwide.12–14
Despite the decline in prevalence, major challenges exist in all countries, including Brazil, regarding the detection of chronically infected patients. Within this context, the question always arises what the best strategy is for detecting possible carriers of the virus. In the present study, two strategies were applied simultaneously: screening at older age (>45 years) and screening at emergency unit and outpatient clinics, places where patients are more likely to have been exposed to parenteral transmission since they often have chronic diseases and are more frequently submitted to invasive procedures and nosocomial transmission.15
In the present study, all identified patients with HCV infection had relevant risk factors: transfusion, intravenous drug use, or sexual exposure. Risk-based testing can identify about 79–99% of individuals infected with HCV.16,17 However, this strategy has some limitations that make it less effective in the screening process, since it depends on adequate and complete anamnesis. Only 58-63% of health professionals ask patients about known risk factors for HCV, a fact that renders this method vulnerable.18 Nevertheless, it is an important strategy considering the results of another study involving 3250 patients from a primary health unit in which 27.8% exhibited at least one risk factor for virus transmission. The study identified that HCV positivity was higher in the group with known risk factors (6.8%) when compared to the group without risk factors (2.2%).16
Regarding age, national and international studies have shown that the prevalence of hepatitis C is higher in the population above 40 years of age compared to other age groups. This fact has encouraged different screening strategies based on age categories, such as baby boomers (individuals born between 1945 and 1965).15 Age above 45 years was used in the present study as recommended by the Brazilian Medical Association in 2015.
The birth-cohort screening strategy appears to be even more efficient than that based on the measurement of alanine aminotransferase (ALT), an enzyme that is altered in chronically infected patients. A study using ALT as the risk variable for a supposed diagnosis of HCV compared to birth-cohort screening found that the latter is superior to the altered ALT approach. Using the birth cohort testing strategy, about 85.4 million people would be tested for anti-HCV and about 2.8 million anti-HCV-positive individuals would be identified, with a sensitivity of 76.6%. On the other hand, the ALT strategy would result in the testing of 21.5 million adults and identification of 1.8 million anti-HCV-positive people, with a sensitivity of 50.0%.19
The strategy of urgency and emergency units screening is based on international studies that showed a high prevalence of HCV in this population,20–22 ranging from 7.3% to 13.8% in some studies, suggesting emergency departments as strategical sites for the detection of HCV.20–23 According to a study conducted with this population, this strategy was a valid approach for identifying patients without known risk factors and who do not belong to any given age group.23
In the United States the number of new cases of HCV infection is increasing among young population.24 Owing to these increases in this age group, the Centers for Disease Control and Prevention (CDC), the US Preventive Services Task Force (USPTF), and the American Association for the Study of Liver Diseases – Infectious Diseases Society of America (AASLD–IDSA) have all endorsed universal screening for HCV in all adults with more than 18 years of age.11 In Brazil the spread use of injected drugs is not a problem nowadays and probably the younger population will show a very low prevalence of HCV. In fact, a study with a large number of young men from Brazilian army showed a prevalence of 0.18% of anti-HCV positivity.25 The strategy of screening all individuals with more than 18 years in Brazil should be evaluated with caution and based on cost-effectiveness studies.
The present study was unable to establish the most adequate strategy for the identification of HCV carriers, also because of the absence of a control group, a limitation of this study. However, other studies suggest the recommendation of the birth-cohort screening, associated or not with other measures such as urgency or emergency units screening, as well as screening individuals with risk factors and a history of parenteral transmission.26,27 In any case, the strategy adopted in this study was able to identify a prevalence of 0.66% of anti-HCV positive patients, a number remarkably close to that observed in more recent studies in our country. Regardless of the strategy adopted, it is important to note that the prevalence of HCV-infected individuals is low in Brazil, even in these possibly more exposed groups. This fact should be taken into consideration when adopting health policies for the identification of carriers of the virus in the country.
In conclusion, in this study carried out at a high-complexity hospital and involving a population of a specific age group for identifying possible infected individuals, the prevalence of hepatitis C was low but in accordance with recent data for Brazilian population, suggesting that this strategy is useful and should be adopted in hospitals with similar characteristics. Furthermore, all identified HCV-positive cases exhibited relevant risk factors, which suggests the need for reflecting on the best and most cost-effective screening strategy to identify HCV-infected individuals in Brazil.
Financial supportNational Council for Scientific and Technological development (CNPq), Brazil, through the Postgraduate Program.
Conflicts of interestThe authors declare no conflicts of interest.