Osteoporosis represents one of the most frequent comorbidity among HIV patients. The current standard method for osteoporosis diagnosis is dual-energy X-ray absorptiometry. Calcaneal quantitative ultrasound can provide information about bone quality. The aims of this study are to compare these two methods and to evaluate their ability to screen for vertebral fracture.
MethodsThis cross-sectional study was conducted in HIV patients attending the Clinic of Infectious and Tropical Diseases of Brescia during 2014 and who underwent lumbar/femoral dual-energy X-ray absorptiometry, vertebral fracture assessment and calcaneal quantitative ultrasound. The assessment of osteoporosis diagnostic accuracy was performed for calcaneal quantitative ultrasound and for vertebral fracture comparing them with dual-energy X-ray absorptiometry.
ResultsWe enrolled 73 patients and almost 48% of them had osteoporosis with at least one of the method used. Vertebral fracture were present in 27.4%. Among patients with normal bone measurements, we found vertebral fracture in proportion between 10% and 30%. If we used calcaneal quantitative ultrasound method and/or X-ray as screening, the percentages of possible savable dual-energy X-ray absorptiometry ranged from 12% to 89% and misclassification rates ranged from 0 to 24.6%. A combined strategy, calcaneal quantitative ultrasound and X-Ray, identified 67% of patients with low risk of osteoporosis, but 16.4% of patients were misclassified.
ConclusionsWe observed that patients with osteoporosis determined by calcaneal quantitative ultrasound and/or dual-energy X-ray absorptiometry have higher probability to undergo vertebral fracture, but neither of them can be used for predicting vertebral fracture. Use of calcaneal quantitative ultrasound for screening is a reasonable alternative of dual-energy X-ray absorptiometry since our study confirm that none strategy is clearly superior, but both screen tools must be always completed with X-ray.
Osteoporosis is defined as “a skeletal disorder characterized by compromised bone strength predisposing to an increased risk of fracture; bone strength reflects the integration of two main features: bone density and bone quality”.1 According to the World Health Organization (WHO) criteria the diagnosis of osteoporosis is based on bone mineral density (BMD) 2.5 standard deviation (SD) or more below the young adult mean (a T-score<2.5 SD).2 A T-score within the normal range is −1.0 SD or above, while a T-score between −1.0 and −2.5 SD characterizes low bone mass, referred to as osteopenia. The current standard method for assessment of BMD is dual energy X-ray absorptiometry (DXA) of the hip and/or spine, and actually the T-score values for osteoporosis and osteopenia diagnosis are validated by WHO only when measured by DXA. Despite osteoporosis-related fractures be a major health issue, osteoporosis remains underdiagnosed.3–5
The association between HIV infection and osteoporosis has been investigated in many studies. A meta-analysis reported the prevalence of osteoporosis and osteopenia in HIV-infected individuals to be as high as 15% and 30%, respectively, corresponding to an odds ratio of 3.7 for osteoporosis and 6.4 for reduced BMD compared to HIV-uninfected individuals.6 The pathogenesis of bone reabsorption is multifactorial.
There are general factors that predispose to osteopenia/osteoporosis, such as gender, age, low body weight, malnutrition, sedentarism, lifestyle factors such as smoking, alcohol, hypogonadism, glucocorticoid, and lipodystrophy.7 Moreover, in the HIV population, there is a direct viral influence that contribute to generate and maintain this process. Indeed, HIV was demonstrated to play a role in increasing apoptosis of osteoblasts and in decreasing their activities and differentiation; on the other hand, it promotes osteoclastic activity and differentiation.8 Last but not least, antiretroviral therapy (in particular NRTI and PI drugs) with his side effects plays an important role in this process.8
Several studies have shown that patients with chronic HIV-infection have an increased risk for fracture compared to non-HIV infected controls.9–11 Patients with HIV-infection have an increased risk of vertebral fractures (VF) due to osteoporosis and BMD assessed at any site fails to predict the risk of VF: 25% of patients with osteopenia and 12% of patients with normal BMD, according to DXA results, showed at least one previously undetected VF.12
Considering that bone strength reflects integration of bone density and bone quality, these findings suggest that bone quality may be as important as bone density in HIV-infected patients.
DXA provides information on mineralization status but does not sheds no light on bone quality.
Quantitative ultrasound (QUS) method can integrate this information. Laboratory investigations have indeed demonstrated that QUS measurements of excised bone specimens are influenced by material properties and structural bone characteristics. In addition, ultrasound velocity has been used extensively to characterize the elastic properties of trabecular and cortical bone.13,14 Taken together, these findings suggest that QUS, unlike BMD, could possibly evaluate bone quality, especially microarchitecture, and therefore be useful for assessing fracture risk.15 Most studies aimed to evaluate the role of the heel QUS in osteoporosis diagnosis were performed in elderly osteoporotic woman16; other studies have considered populations characterized mainly by poor bone quality.17,18
The aim of this study was to investigate the HIV-infected population at risk of osteoporosis, according with the current guidelines for osteoporosis,19 measuring the BMD by calcaneal quantitative ultrasound (QUS), the BMD by DXA at femoral neck (FN-DXA) and lumbar spine (L-DXA), and detecting the presence of osteoporotic VF by thoracic-lumbar X-ray, evaluating the ability of these techniques to screen for osteoporosis in patients with HIV chronic infection.
Materials and methodsThis cross-sectional study was conducted at the Clinic of Infectious and Tropical Diseases of Brescia (north of Italy). We included HIV-infected patients attending our HIV-outpatient Clinic during 2014 who underwent BMD measurement (at lumbar spine and hip) and vertebral fractures assessment at the Endocrinology outpatients Clinic; and QUS, in our Clinic. Patients with hypophosphatemia, hyperparathyroidism, postmenopausal women and male patients older than 50, as indicated in the current HIV infection management guidelines were recruited for the study. We excluded patients with previous osteoporosis diagnosis and self-reported fractures or traumas. Demographic data were collected from the clinical charts. At first visit to our center all patients provided written informed consent to allow inclusion of their clinical and biological data to be used for scientific purpose. BMD and QUS measurements and lateral spine X-ray were performed in all study participants. BMD of the femoral neck and lumbar spine were measured using DXA (Lunar Prodigy 8743; GE Medical Systems, Madison Wisconsin). The ultrasound scanning of calcaneus of the left foot was performed with Hologic Sahara device (Hologic, Inc., Waltham, MA, USA). The ultrasound variables broadband ultrasound attenuation (BUA), speed of sound (SOS), and the respective calculated stiffness value (defined as 0.28×SOS+0.67×BUA 420) or the QUS index (QUI; defined as 0.41×SOS+0.41×BUA 571) were collected. QUS is used to obtain an estimate of the heel BMD in g/cm2. For the assessment of vertebral fractures (VF), anteroposterior and lateral X-ray examinations of the thoracic and lumbar spine were performed and centrally digitized. A quantitative morphometric assessment of VF in T4–L4 was performed using a dedicated morphometry software (Spine-X analyzer; ICAM Diagnostics, Milan, Italy).
Correlation coefficients were applied to evaluate the relationship between BMD and T-score among the sites and techniques and determined using the Pearson's correlation r coefficient.
Logistic regression analysis was performed, with the presence of VF as dependent dichotomic variable and T-score values. Results are expressed as the odds ratio of vertebral fracture with 95% confidence interval (CI). Receiver operating characteristic (ROC) analysis was performed, and we calculated the areas under the curve (AUC) in order to determine the ability of QUS and DXA (lumbar spine and femoral neck) to discriminate subjects with or without vertebral fractures.
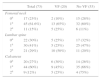
ResultsThe study population consisted of 73 HIV-infected patients aged 34–73 years (mean 54.8 years), most of them were male (76.7%). Patients’ characteristics are summarized in Table 1. The number of patients classified as “osteoporotic” varied according to the method of measurement used and site evaluated. Almost 48% (n=35/73) of patients were diagnosed as osteoporotic by at least one of the methods. According to the WHO definition based on T-score values, the proportion of patients with osteoporosis at femoral neck, lumbar spine (measured by DXA) and calcaneus (measured by QUS) were 15%, 29%, and 12%, respectively, while patients with osteopenia were 61.6%, 41%, and 60%, respectively. Osteoporotic VF were present in 27.4% (n=20/73) of patients. The mean age of patients with (58.15 years) and without VF (53.57 years) was significantly different (p=0.04). In contrast, no statistically significant differences were found for the following variables: gender, BMI, cigarette smoking, HCV or HBV infection, diabetes mellitus, hypophosphatemia, or hyperparathyroidism. Interestingly, among patients with normal bone measurements (T-score>−1.0 SD), osteoporotic vertebral fractures were found between 10% and 30% of patients depending on the method used (Table 2).
Patients’ characteristics.
| Variable | n (%) |
|---|---|
| Total: 73 (100%) | |
| Male gender | 56 (76.7%) |
| Postmenopausal women | 10 (58.8%) |
| Age, years [mean (min−max)] | 54.8 (34–73) |
| BMI [mean (min−max)] | 25.2 (16.6–39.9) |
| <25 | 35 (47.9%) |
| 25.0–29.9 | 28 (38.3%) |
| ≥30 | 10 (13.7%) |
| Cigarette smoking | 53 (72.6%) |
| HCV-Ab positive | 40 (54.8%) |
| HBsAg positive | 2 (2.7%) |
| Diabetes mellitus | 1 (1.3%) |
| Hypophosphatemia (<2.4mg/dl) | 17 (23.3%) |
| Male older than 50 years | 39 (53.4%) |
| Hyperparathyroidism | 22 (30.1%) |
BMI, body mass index.
Vertebral fractures (VF) distribution according to presence of osteopenia or osteoporosis at several sites as defined by WHO.
| Total (73) | VF (20) | No VF (53) | |
|---|---|---|---|
| Femoral neck | |||
| 0a | 17 (23%) | 2 (10%) | 15 (28%) |
| 1a | 45 (61.6%) | 13 (65%) | 32 (60%) |
| 2a | 11 (15%) | 5 (25%) | 6 (11%) |
| Lumbar spine | |||
| 0a | 22 (30%) | 5 (25%) | 17 (32%) |
| 1a | 30 (41%) | 5 (25%) | 25 (47%) |
| 2a | 21 (29%) | 10 (50%) | 11 (20%) |
| Calcaneus | |||
| 0a | 20 (27%) | 6 (30%) | 14 (26%) |
| 1a | 44 (60%) | 9 (45%) | 35 (66%) |
| 2a | 9 (12%) | 5 (25%) | 4 (75%) |
Table 3 shows the mean T-score at femoral neck, lumbar spine (measured by DXA), and calcaneus (measured by QUS) in patients with and without vertebral fractures. p-Values were not statistically significant when comparing those with and without VF.
Mean T-score according to the method and site in patients with and without vertebral fractures (VF).
| Mean (SD) | VF | NO VF | p-Value | |
|---|---|---|---|---|
| Mean (max; min) | Mean (max, min) | |||
| Femoral T-score | −1.54 (0.89) | −1.42 (−4.6; +0.3) | −1.87 (−3.2; +0.5) | 0.051 (NS) |
| Lumbar spine T-score | −1.54 (1.32) | −1.38 (−4.3; +1.1) | −1.97 (−4.3; +0.6) | 0.082 (NS) |
| Calcaneal T-score | −1.39 (1.08) | −1.30 (−3.5; +3.0) | −1.62 (−3.4; +0.8) | 0.316 (NS) |
NS, not statistically significant.
When T-score was considered as continuous variable, the Spearman's coefficient between femoral neck and lumbar T-score was 0.47, between femoral neck and calcaneus was 0.55, and between lumbar and calcaneus was 0.43 (p<0.001 for each comparison). The association between T-score and VF was assessed either as stratified or continuous values (Table 4). Patients with osteoporosis according to QUS and/or DXA at any site had higher probability to have undergone vertebral fractures, although not statistically significant for T-score<−2.5 [OR=3.09 (0.83–11.51) at femoral neck; OR=2.92 (0.57–14.82) at lumbar spine; or OR=6.25 (0.94–41.52) at calcaneus].
Association between stratified or continuous T-score of different sites and vertebral fractures (VF).
| T-score | Odds Ratioa (95% CI) | p-Value | AUC (95% CI) |
|---|---|---|---|
| FemoralT-score | |||
| Continuous | 2.44 (0.99–6.03) | 0.054 | 0.63 (0.51–0.75) |
| Stratified | |||
| 0 | Ref | 0.67 (0.53–0.80) | |
| 1 | 0.68 (0.17–2.71) | 0.585 | |
| 2 | 3.09 (0.83–11.51) | 0.092 | |
| Lumbar spineT-score | |||
| Continuous | 1.89 (0.93–3.84) | 0.077 | 0.63 (0.48–0.78) |
| Stratified | |||
| 0 | Ref | 0.63 (0.49–0.76) | |
| 1 | 0.6 (0.18–2.00) | 0.406 | |
| 2 | 2.92 (0.57–14.82) | 0.197 | |
| CalcanealT-score | |||
| Continuous | 1.45 (0.62–3.39) | 0.390 | 0.55 (0.40–0.70) |
| Stratified | |||
| 0 | Ref | 0.63 (0.51–0.75) | |
| 1 | 3.05 (0.61–15.24) | 0.175 | |
| 2 | 6.25 (0.94–41.52) | 0.058 | |
0, normal BMD; 1, osteopenia; 2, osteoporosis.
In this study we investigated different methods for detection of osteoporosis and osteopenia in a cohort of HIV infected patients. In particular, we compared the gold standard DXA with calcaneal QUS, an ultrasound based-strategy. Moreover, we evaluated the presence of osteoporotic vertebral fractures in patients who underwent bone structure screening. There was a moderate correlation between T-scores measured by DXA and by QUS, but QUS as well as DXA were unable to discriminate between patients with and without VF, as previously reported in other studies.20,21 Indeed, VF may also occur with normal or modestly reduced BMD measured by DXA and with good bone quality tested by calcaneal QUS.22 In a previous study, we found a high prevalence of VF (27%) in HIV-infected patients while BMD assessed by DXA (both at femoral neck and lumbar spine) failed to demonstrate osteoporosis.12 The present data confirm previous data and suggest that neither QUS or DXA can be used in HIV-infected patients for predicting VF.23–25 In this context, when evaluating HIV patients, the application of FRAX score is very useful for the assessment of fracture risk, because this tool gives the 10-year probability of a major osteoporotic fracture and it can guide decisions about who to screen.26 However, FRAX score has been validated only in the general population, and it can underestimate fracture risk in HIV-infected patients, just like other useful risk assessment tools such as Framingham score,27 which is not studied in the HIV population.
WHO defines osteoporosis based upon DXA measurements.1 In fact, this method remains the most widely used for measuring BMD and T-score based on DXA measurements are the only validated for osteoporosis/osteopenia diagnosis. DXA provides accurate measurements at clinically relevant sites, i.e. those with major clinical consequences in case of a fracture; on the other hand, the major disadvantages of DXA are non-portability (the machine is large), relatively expensive compared to alternative peripheral technologies (especially if performed in low-income countries), and the use low-dose radiation.28,29 Alternative techniques for evaluation of bone status at peripheral sites have been developed. Among them quantitative ultrasound measurement of the heel is considered one of the best alternative method currently available for the assessment of fracture risk.30–34 An advantage of this technique is the possibility of detecting structural bone characteristics (elastic properties, trabecular orientation) thus enabling a better quantification of bone quality. Indeed, it is portable, cheaper, and easier to perform in comparison with DXA.
As previously reported, there are few studies comparing QUS and DXA methods that showed that QUS parameters correlate well with BMD of the same local areas assessed with DXA.35,36 However, the crux of the matter is whether these QUS parameters predict the predisposition for skeletal fractures. Integrated information obtained by QUS can represent a reasonable alternative because bone structural quality may conflict with measurement of BMD which only detects bone density.16,37
Although QUS and DXA measure different bone characteristics (quality and quantity, respectively) a discrete correlation between T-score has been described in different populations of HIV-uninfected persons.38,39
Recently, Borderi et al.8 confirmed this correlation also in HIV-infected people. Compared to Borderi's findings, the r coefficient found in our study was slightly higher probably due to differences between the two study populations: our cohort was older and had higher risk of osteoporosis according to guidelines compared to patients included in Borderi's study.8
The major limitation of our study was the small size of the cohort of patients included. Further studies are needed to analyze the potential of this alternative and promising technique.
Given the relatively small difference between various osteoporosis screening strategies (calcaneal QUS or DXA) found in our study and since neither strategy was clearly superior, patient preferences should play an important role in the choice of the screening strategy. For patients with limited access to DXA or those who prefer not to travel for DXA screening, using QUS for screening is a reasonable alternative. Both screening strategies must always be combined with thoracic X-ray.
Conflicts of interestThe authors declare no conflicts of interest.