Staphylococcus aureus has been recognized as an important pathogen associated with inpatients and community infections. Community-acquired methicillin-resistant S. aureus (CA-MRSA) infections commonly present as skin and soft-tissue infections (SSTIs). Treatment often includes incision and drainage with or without adjunctive antibiotics.
ObjectivesThis study aimed to identify CA-MRSA infections both phenotypically and genotypically, to determine their spectrum of antibiotic resistance, and to establish the best scheme for molecular distinction between hospital-acquired MRSA (HA-MRSA) and CA-MRSA by staphylococcal cassette chromosome mec (SCCmec) typing and detection of Panton Valentine leukocidin (PVL).
Materials50 swabs, from skin and soft tissue of infected lesions of outpatients attending the dermatology department of the Medical School, Alexandria University, were collected. Additionally, a nasal swab was taken from every participant.
MethodsCollection of swabs from the infected skin and soft tissues, followed by laboratory testing to phenotypically and genotypically identify MRSA. Also, nasal swabs were taken from every patient to identify MRSA colonization.
ResultsStaphylococcus aureus strains were identified in 38 (76%) of the 50 clinical isolates. 18 (47.37%) out of the 38 S. aureus strains were resistant to oxacillin and cefoxitin discs, were penicillin binding protein 2a (PBP2a) producers, and were initially diagnosed as MRSA. All of the 18 strains were definitively diagnosed as MRSA by mecA gene detection using real time PCR, while only six (33.33%) strains were PVL positive. Using the sets of primers of Zhang et al.: nine (50%) out of the 18 CA-MRSA strains were SCCmec type V, and one (5.56%) was SCCmec type IVc. Then, using the set of primers by Oliveira et al., two (25%) out of the eight untypable MRSA strains were found to be SCCmec type IV, and six (75%) remained untypable.
ConclusionsCA-MRSA must be considered when treating skin and soft tissue infections, especially in developing countries. Empirical use of agents active against CA-MRSA is warranted for patients presenting with serious SSTIs.
Among staphylococcal species, Staphylococcus aureus is the most pathogenic organism. The spread of antibiotic resistance among strains of S. aureus is a major concern in the treatment of staphylococcal infections. It is well known that the organism acquires resistance soon after the introduction of new antibiotics. Methicillin was developed in 1960 for the treatment of multi-drug resistant S. aureus. However, in the same year, methicillin-resistant S. aureus (MRSA) was discovered.1–3MRSA infections have usually been associated with exposure to health care settings, but they have recently been identified in people without traditional risk factors. These infections have been called community-acquired or community-associated MRSA (CA-MRSA).1 Most cases have been associated with skin and soft-tissue infection (SSTI), and have been reported among selected populations, including correctional facility inmates, homosexual men, and athletes.4–6
CA-MRSA was first described in the 1980s in drug users and in the 1990s the epidemic emerged in persons without antecedent healthcare exposure. It became a major concern worldwide. CA-MRSA is responsible for a wide array of infections from superficial skin infections to life-threatening diseases.5,7
The accurate and early determination of methicillin resistance is of key importance in the prognosis of infections caused by S. aureus. Although multiple methods of detection of this resistance have been developed, identification of the mecA gene is the most reliable method of detecting MRSA isolates.7,8
Asymptomatic carriers usually act as reservoirs for infection during outbreaks caused by S. aureus,9,10 and colonization is a predisposing factor for infection with CA-MRSA.11,12
CA-MRSA has been reported to carry the loci for Panton-Valentine leukocidin (PVL), in high frequency in association with the type IV and type V SCCmec.13–15 PVL is a virulance determinant factor for MRSA.
The aim of this study was to identify CA-MRSA infections both phenotypically and genotypically, to determine their spectrum of antibiotic resistance, and to establish the best method for molecular distinction between hospital acquired MRSA (HA-MRSA) and CA-MRSA by SCCmec typing and detection of PVL.
MethodsPatientsFifty patients suffering from skin and or soft tissues infections were chosen according to the criteria of the Centers for Disease Control and Prevention (CDC), which defined CA-MRSA infection as: identification of MRSA in a patient with signs and symptoms of infection, either in the outpatient setting or within 48hours after admission to a hospital, with no history of MRSA infection or colonization; no history of admission to a hospital or a nursing home during the previous year; and absence of dialysis, surgery, permanent indwelling catheters, or medical devices that pass through the skin to the body.16–18
All the patients attended the dermatology department of the Faculty of Medicine, Alexandria University.
Demographic data of the patientsAge: from 5 to 40 years.
Gender: 40 males and 10 females.
Occupations: 10 school children, 30 military personnel (one family member of military personnel); 10 patients were adults working in bad hygiene and crowded conditions.
Residence: 30 patients from overcrowded bad hygienic condition from rural area, 20 patients from urban area. Type of skin lesions: eight patients had impetigo, ten had boils, seven had carbuncle, three had infected pediculosis, seven had infected scabies, five had infected tinea capitis, and ten had infected wounds. Sites of skin lesions: eight on back of neck, seven on the groin, five on the buttock, five on the armpit, four on the beard area of males, three on the scalp, eight on the abdomen, four on the back, three on the leg, and three on the foot.
MethodSwabs from infected skin and soft tissue lesions were taken by inserting a swab into the lesion and rotating it for a count of five seconds. The swab was then placed carefully into its container, labeled as skin or soft tissue, and patient details were completed clearly before being inserted into a microbiology form.
Nasal swabs were taken by inserting a swab into a nostril, and rotating it for a count of five seconds before repeating the process with the other nostril. It is not necessary to insert the swab very far into the nasal passage. The swab was then placed carefully into its container, labeled “nasal swab”, and patient details were completed clearly before being inserted into a microbiology form.
The swabs were obtained after the signing of an informed consent (in the case of children, their parents signed the informed consent). The study was approved by the Research Ethics Committee of the Alexandria Faculty of Medicine.
Identification of staphylococcal isolatesAll samples were inoculated on blood agar and MacConkey agar plates. Staphylococcal isolates were identified by their colonial appearance. Colonies suspected as staphylococci were Gram-stained and tested by catalase, oxidase, slide coagulase, and tube coagulase test.19,20
Antibiotic susceptibility testingSusceptibility profile of staphylococci was determined by disc diffusion method including oxacillin and cefoxitin discs.21,22
Detection of penicillin binding protein 2a (PBP2a) latex agglutination testThe PBP2a latex agglutination test was performed to detect the presence of PBP2a, which is responsible for methicillin resistance.23
Polymerase chain reaction (PCR) staphylococcal DNA extractionUsing SYBR Green 1 technique.24–26
Procedures:
- 1.
Staphylococcal DNA extraction: staphylococcal isolates were subcultured overnight at 37° C on blood agar media. Some colonies were emulsified in 200μL sterile distilled water to produce a heavy suspension. The bacterial suspension was heated at 100°C for 15min. The suspension was then centrifuged at 14.000rpm (Hettich zentrifugen) for 5min.
- 2.
Amplification protocol: each PCR tube contained the following:
▪12.5μL SYBR Green universal PCR master mix 2-fold concentrate were added to the reaction mixture.
▪6.5μL PCR grade water, bringing the reaction volume to 25 (L.
▪0.5μL forward primer.
▪0.5μL reverse primer.
▪5μL DNA extract.
A negative control was prepared by adding the same contents to a tube with water instead of extract.
- 3.
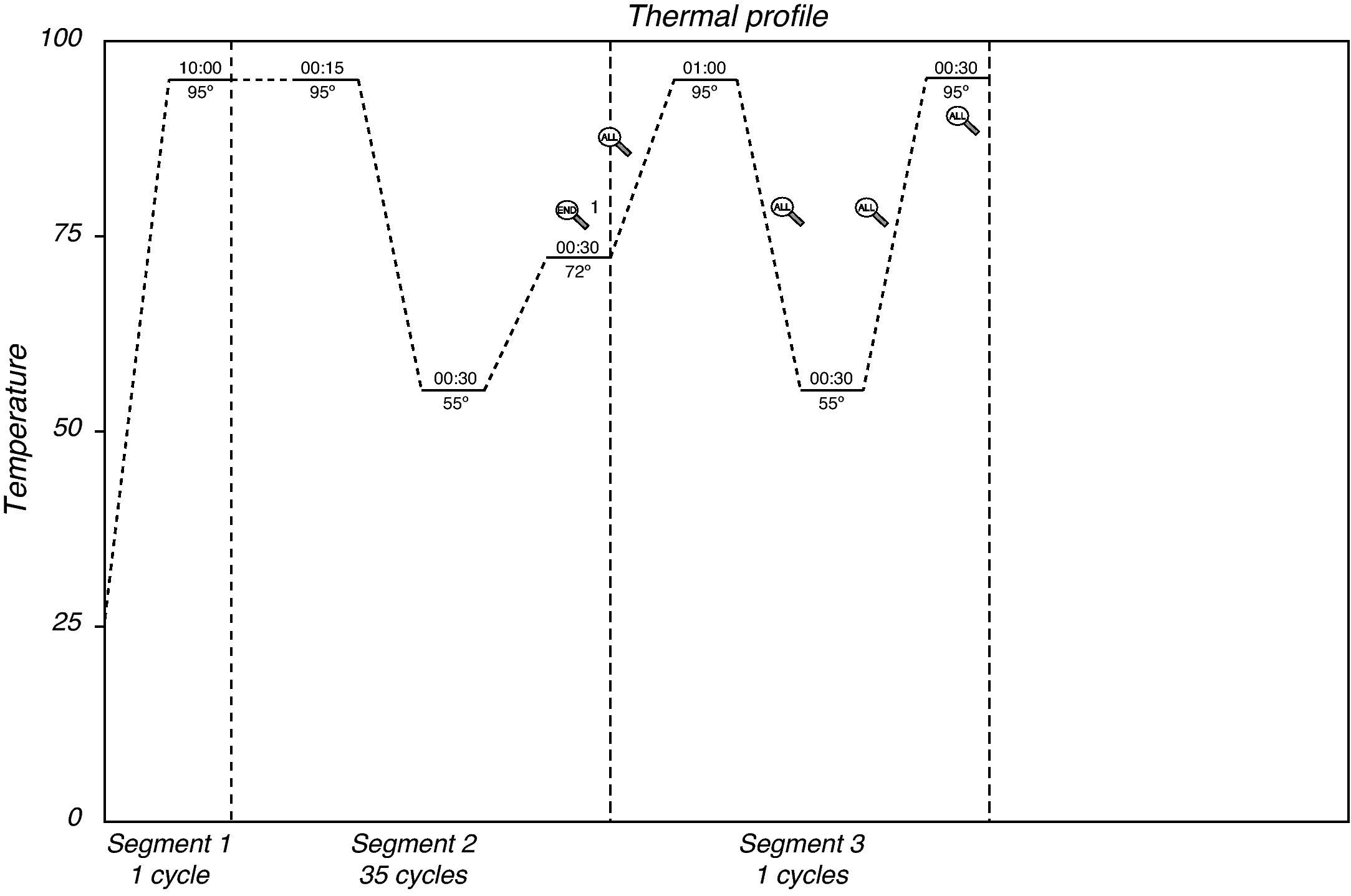
The tubes were placed in the thermal cycler for amplification according to the following thermal profile (Fig. 1).
Real time PCR was used to determine the occurrence of PVL as a virulence factor in CA-MRSA. The presence of PVL genes in selected MRSA isolates was assessed by using PCR as previously described by Lina et al.27
The characterization and subtyping were performed according to Zhang et al.,26 and rapid identification of structural types and variants of the mec element in MRSA were performed according to Oliveira et al.28
ResultsS. aureus was identified by slide and tube coagulase tests in 38 (76%) out of the 50 clinical isolates. 29 (76.31%) out of the 38 S. aureus isolates were mannitol fermenters. The antibiotic sensitivity of CA-MRSA ranged from 22.22% for ceftriaxone to 94.44% for imipenem and rifampicin. 18 (47.37%) out of the 38 S. aureus isolates were resistant to oxacillin and cefoxitin discs and were initially diagnosed as MRSA.
All the 18 strains were finally diagnosed as MRSA by detection of mecA gene using real time PCR using SYBR Green 1 technique. All 18 MRSA strains (100%) were PBP2a producer. Out of the 18 CA-MRSA, ten were typable by the set of primers by Zhang et al.: nine (50%) belonged to SCCmec type V, and one (5.56%) to SCCmec type IVc.
Using Oliveira et al.28 set of primers, two (25%) of the eight untypable MRSA strains were found to be type IV, and six (75%) remained untypable. Out of the 18 MRSA isolates, only six (33.33) were PVL positive.
Regarding skin lesions CA-MRSA isolates were recovered from abscess, furuncles and carbuncles, and from infected surgical wounds. Regarding nasal swabs: nasal swabs were positive for CA-MRSA in all cases infected with CA-MRSA. Regarding the site of the lesions CA-MRSA isolates were recovered from back of neck, groin, buttock, armpit, and beard area of males. Most of cases diagnosed as CA-MRSA came from overcrowded and rural areas.
DiscussionIn the present study, a total of 50 swabs were collected from skin and soft tissue of infected lesions in outpatients attending the dermatology department of the Faculty of Medicine, Alexandria University, from October 2009 to December 2010. Nasal swabs were also taken from every participant.
In the present study, 50 isolates were collected from different skin infections. 42 (84%) were identified as Staphylococcus spp., four (8%) as Streptococci spp., and four (8%) as Proteus spp. 38 (90.48%) out of the 42 staphylococci were diagnosed as S. aureus. Similar results were found by Abdallah et al.,29 Mohanty et al.,21 and Moet et al.30
The accurate and early determination of methicillin resistance is of key importance in the prognosis of infections caused by S. aureus. Although multiple methods of detection of this resistance have been developed, they are often too slow or not sufficiently sensitive or specific to ensure appropriate treatment of the MRSA-infected patients.19,20
Identification of the mecA gene is the most reliable method for detecting MRSA isolates; however, not all laboratories can include molecular biology techniques in their routine clinical practice. For this reason, it is essential to evaluate phenotypic techniques for MRSA detection in a rapid and accurate manner, in order to ensure correct antibiotic treatment and to avoid the spread of MRSA isolates in the environment.19,20
Methods based on disc diffusion, as well as microdilution with oxacillin, are often not entirely reliable at detecting some strains that harbor the mecA gene.31
In the present study, out of the 38 S. aureus strains isolated from the different skin lesions, 18 (47.37%) were positive for the mecA gene. All strains were resistant to both cefoxitin disc and oxacillin disc (sensitivity 100%).
Swenson et al. assessed cefoxitin disc diffusion for predicting mecA-mediated oxacillin resistance in S. aureus and found that the sensitivity and specificity of the test were 98% and 100%, respectively, for S. aureus. They demonstrated that oxacillin MIC, oxacillin disc diffusion tests, and cefoxitin disc diffusion are essentially equivalent in performance for both sensitivity and specificity.32
Velasco et al. found that three (5.9%) out of the 51 clinical strains that were positive for the mecA gene yielded false negative results with the oxacillin disc diffusion, (sensitivity 94.1%). They explained the lower sensitivity by the absence of, or reduced expression of, the mecA-encoded protein, PBP2a. The results of their study confirmed that antibiotics that are able to induce the expression of methicillin resistance, such as cefoxitin, are the most appropriate for detecting MRSA isolates, as all mecA-positive isolates were detected with the cefoxitin disc, with a sensitivity of 100%.31
Identification of MRSA is more accurate either by directly detecting the gene encoding the methicillin resistance determinant (mecA) or its product (PBP2a).22,23 This is in accordance with Cavassini et al.33 and Sakoula et al.34 In this study, the 18 MRSA strains were PBP2a producers.
In the present study, 27.78% of the strains were found to be resistant to gentamycin, 11.11% were found to be resistant to co-trimoxazole, and 22.2% to ofloxacin.
Both CA-MRSA and HA-MRSA are resistant to traditional anti-staphylococcal beta-lactam antibiotics. CA-MRSA isolates tend to be more susceptible to other antibiotics (including sulfa drugs and tetracyclines) than HA-MRSA isolates, and their narrow spectrum of resistance is solely due to determinants harboured on genetic elements present on the SCCmec. HA-MRSA may contain resistance elements for numerous antibiotic classes, including macrolides, lincosamides, aminoglycosides, fluoroquinolones, tetracyclines, and sulfonamides.7
Kaplan noted that most of the CA-MRSA isolates are susceptible to vancomycin, gentamycin, rifampicin, co-trimoxazole, clindamycin, doxycycline, and linezolid.35
In the present study, 100% of the CA-MRSA isolates were resistant to fusidic acid. The prolonged use of fusidic acid as topical monotherapy for chronic skin conditions appears to have resulted in the emergence of resistance among S. aureus in some countries, making this agent less active both for topical and systemic therapy. Resistance to this antibiotic has also been detected in community-acquired methicilin-susceptible S. aureus (CA-MSSA) strains, as well as CA-MRSA strains in other European countries.36
SCCmec typing is one of the most important molecular tools available for understanding the epidemiology and clonal strain relatedness of MRSA, particularly with the emerging outbreaks of CA-MRSA occurring on a worldwide basis.19,20
In the present study, the mec gene complex of the 18 MRSA strains could not be classified as class A or B using Zhang et al.26. Conversely, by using Zhang et al. specific primers for identification of SCCmec types and sub-types I, II, III, IVa, IVb, IVc, IVd, and V, nine (50%) of the 18 MRSA strains were classified as SCCmec type V, one (5.56%) was classified as type IVc, and eight (44.44%) were untypable.
In the present study, using Oliveira et al. primers, two out of the eight untypable MRSA strains were classified as SCCmec type IV, while six remained untypable.
Similar results were reported by Hanssen et al., who found strains that did not fit into the SCCmec typing, which were designated as possibly new SCCmec types.37
In the present study, three (16.67%) out of the 18 CA-MRSA strains were classified as SCCmec type IV using the schemes of Zhang et al. and those of Oliveira et al.26,28
There has been much interest in PVL, due to its involvement in severe disease among children and young adults with no known exposure to healthcare establishments. CA-MRSA has been reported to carry the loci for PVL at high frequency, and to be associated with the SCCmec type IV.27
In the present study, six (33.33%) out of the 18 MRSA isolates were PVL positive while among the 20 MSSA isolates only three (15%) were PVL positive. Among the six PVL positive MRSA isolates, three were SCCmecV, one SCCmecIV, and two were untypable.
In the present study, PVL positivity was not related to SCCmec typing since one third of the PVL positive strains were untypable while only 33.3% of each of type IV and V were PVL positive.
However, five of the PVL positive strains were sensitive to the majority of the tested antibiotics, indicating their community-acquired origin.
More recently, cases of community-acquired pneumonia due to PVL-positive S. aureus have been reported in France, Sweden, the Netherlands, and the United Kingdom. In addition, the PVL genes have been identified as a stable marker of CA-MRSA strains worldwide.27,38
When isolates were categorized according to type of staphylococcal infection, the PVL genes were strongly associated with skin and soft tissue infections, such as abscesses, skin lesions, and boils (furuncles).27,38
Conversely, PVL genes were seldom found in the general S. aureus population, a result in concordance with a Swedish study that showed a low prevalence of PVL among S. aureus isolates from patients with primary skin infections, pneumonia, and staphylococcal bacteremia.27,38
In the present study, the combination of SCCmec typing and PVL positivity was not sufficient to identify all CA-MRSA isolates, due to the absence of PVL among several CA-MRSA strains, and due to the presence of untypable MRSA.
The combination of phenotypic characteristics in conjunction with certain antibiograms is useful to a degree, but further work is required to find a reliable marker or combination of markers to facilitate the recognition of CA-MRSA. It appears that, to date, there is no unique marker or combination of markers that can substitute for descriptive epidemiology in the recognition of CA-MRSA.
The nasal swab was positive for CA-MRSA in every patient who was positive for CA-MRSA skin and soft tissue infection, demonstrating that colonization is a predisposing factor for infection with CA-MRSA.
Conflict of interestAll authors declare to have no conflict of interest.