Ventilator-associated pneumonia is the most prevalent nosocomial infection in intensive care units and is associated with high mortality rates (14–70%).
AimThis study evaluated factors influencing mortality of patients with Ventilator-associated pneumonia (VAP), including bacterial resistance, prescription errors, and de-escalation of antibiotic therapy.
MethodsThis retrospective study included 120 cases of Ventilator-associated pneumonia admitted to the adult adult intensive care unit of the Federal University of Uberlândia. The chi-square test was used to compare qualitative variables. Student's t-test was used for quantitative variables and multiple logistic regression analysis to identify independent predictors of mortality.
FindingsDe-escalation of antibiotic therapy and resistant bacteria did not influence mortality. Mortality was 4 times and 3 times higher, respectively, in patients who received an inappropriate antibiotic loading dose and in patients whose antibiotic dose was not adjusted for renal function. Multiple logistic regression analysis revealed the incorrect adjustment for renal function was the only independent factor associated with increased mortality.
ConclusionPrescription errors influenced mortality of patients with Ventilator-associated pneumonia, underscoring the challenge of proper Ventilator-associated pneumonia treatment, which requires continuous reevaluation to ensure that clinical response to therapy meets expectations.
Although there have been advances in preventing ventilator-associated pneumonia (VAP), it remains the most prevalent nosocomial infection in intensive care units (ICU).1 VAP impairs patient recovery by increasing length of hospitalization, duration of mechanical ventilation, and hospitalization costs.2 Moreover, VAP is associated with high mortality rates (14–70%), which are higher in infections due to resistant bacteria, inappropriate antimicrobial therapy use, and incorrect antimicrobial prescription or de-escalation therapy.3,4
VAP is often caused by resistant bacteria, which may limit therapeutic options and compromise patient outcomes in clinical practice.5
As VAP is associated with significant morbidity and mortality, the choice of initial empiric treatment should take into account the risk of infections caused by resistant organisms. In addition, proper prescription of antimicrobial therapy should also consider the type, dosage, and duration of drug administration. Despite the availability of guidelines for VAP diagnosis and treatment, therapy still varies significantly between institutions and the occurrence of incorrect therapy prescription is quite high, ranging from 10% to 73%.6,7
The aim of this study was to evaluate factors influencing the mortality of patients diagnosed with VAP, including bacterial resistance, prescription errors, and de-escalation of antimicrobial therapy.
MethodsThis retrospective study reviewed medical records of patients admitted to the adult ICU of the Federal University of Uberlândia (Adult ICU/UFU), between January 1st and July 31st, 2013. The patients included in the study were 18 years or older who were diagnosed with VAP. Diagnosis was based on criteria established by the American Thoracic Society and the Infectious Diseases Society of America,8 including: mechanical ventilation for at least 48h and appearance of new or progressive pulmonary infiltrate on chest radiographs associated with at least two clinical signs and/or laboratory changes suggesting an ongoing infection, including fever (>38°C) or hypothermia (<35°C); leukocytosis (>10,000/mm3) or leukopenia (<4000/mm3); purulent tracheal secretions; and oxygenation changes.
Out of the total of 467 medical records of patients admitted to the Adult ICU/UFU during the study period analyzed, there were 132 cases of VAP in 120 patients, since 12 patients had two episodes of infection. In patients who had more than one episode of VAP diagnosed during the study period, we included only the first identified case of VAP. The study was approved by the Ethics Committee of the Federal University of Uberlândia (protocol number 775.657) and registered as a clinical trials service of the U.S. National Institutes of Health (protocol number 30121978).
Medical records were abstracted to obtain information on patient age, gender, primary diagnosis at admission, comorbidities, prognostic indexes (Acute Physiology and Chronic Health Disease Classification System II [APACHE II] and Simplified Acute Physiology Score III [SAPS III]); causative bacteria identified and sensitivity profiles, the characteristics of antimicrobial prescriptions, and outcome (discharge or death).
Based on data in the medical records, specifics about prescription and administration of antimicrobial therapy were obtained, including whether treatment was administered after having obtained the results of sensitivity profiling using quantitative culture, as well as de-escalation (interruption of antimicrobial treatment or replacement by an antimicrobial with limited-spectrum coverage); escalation (addition of a new antimicrobial or replacement by a broad-spectrum antimicrobial); or maintenance (maintenance of antimicrobial initially prescribed or replacement by an antimicrobial with the same coverage profile).8
Errors in antimicrobial prescription were classified as follows: inappropriate choice (different choice from literature recommendations); errors in loading or maintenance dose (prescription of a higher or lower dose compared to the indicated dose); errors in the interval between doses (higher or lower interval between doses compared to the indicated interval); delay in starting antimicrobial therapy (more than one hour between prescription and administration of the first antimicrobial dose); inappropriate adjustment for body weight (no dose correction based on patient weight); inappropriate adjustment for renal function; errors in treatment duration (prescription for shorter or longer duration than the indicated period). To analyze treatment adequacy based on the literature, we used guideline recommendations for management and health care of adults with nosocomial pneumonia associated with mechanical ventilation from the American Thoracic Society and the Infectious Diseases Society of America.8The Sanford Guide to Antimicrobial Therapy8 were used as standards for decisions about starting time; dose and indicated dosage; and adjustments, when necessary, for weight and renal function.9 Error in starting of antibiotic therapy was defined by the Surviving Sepsis Campaign10 as more than one hour between prescription of the first antibiotic dose by the attending physician and administration to the patient.
Multidrug-resistant bacteria were defined as bacteria resistant to three or more classes of antimicrobials. Gram-positive bacteria were assessed for oxacillin resistance.9 According to local characteristics the resistance profile of the Adult ICU/UFU has been defined as follows: Staphylococcus aureus and Staphylococcus epidermidis sensitive or not to oxacillin (MRSA), Pseudomonas aeruginosa and Acinetobacter baumannii resistant to carbapenems (imipenem and meropenem), enterobacteriaceae (Escherichia coli, Enterobacter spp, Klebsiella pneumoniae spp, Serratia spp) for the production of beta-lactamase extended spectrum (ESBL) and Stenotrophomonas maltophilia resistant to trimethoprim/sulfamethoxazole.
Statistical analysisChi-square test was used to compare qualitative variables. Student's t test was used to compare means between groups of normally distributed quantitative variables.
Multiple logistic regression analysis was used to evaluate mortality independent predictors in the ICU. SPSS Statistics for Windows was used for analysis, and results were considered statistically significant when p<0.05.
ResultsOf patients included in this study, 32% were diagnosed with VAP in the Adult ICU/UFU during the study period. An overall mortality rate of 35% of patients with VAP was observed. The patients were predominantly male (74%), with an average age of 49±19 years, average time of hospitalization of 35±26 days, and average admission APACHE II and SAPS III prognostic index scores of 19.5±7.5 and 61.9±15, respectively.
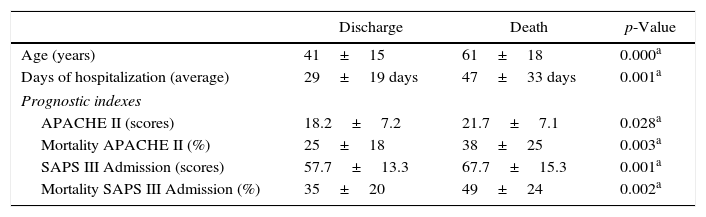
Patient clinical characteristics, prognostic index scores, and discharge or death outcomes in the ICU are shown in Table 1. The mortality rate was higher in older patients and those with higher prognostic index scores. Cardiovascular failure was the most frequent reason for hospitalization (p=0.000). Analysis of comorbidities revealed a significant correlation between death and diabetes (p=0.008), heart disease (p=0.000), and lung disease (p=0.039). The average duration of hospitalization was 38% higher in the group of patients who died (p=0.001) (Table 1).
Clinical characteristics and prognosis based on clinical outcomes of discharge or death among patients diagnosed with ventilator-associated pneumonia.
| Discharge | Death | p-Value | |
|---|---|---|---|
| Age (years) | 41±15 | 61±18 | 0.000a |
| Days of hospitalization (average) | 29±19 days | 47±33 days | 0.001a |
| Prognostic indexes | |||
| APACHE II (scores) | 18.2±7.2 | 21.7±7.1 | 0.028a |
| Mortality APACHE II (%) | 25±18 | 38±25 | 0.003a |
| SAPS III Admission (scores) | 57.7±13.3 | 67.7±15.3 | 0.001a |
| Mortality SAPS III Admission (%) | 35±20 | 49±24 | 0.002a |
| Diagnosis at admission | n (%) | n (%) | |
|---|---|---|---|
| Neurologic | 35 (2) | 13 (11) | 0.131 |
| Trauma | 26 (2) | 2 (2) | 0.001a |
| Respiratory | 6 (5) | 8 (7) | 0.091 |
| Infectious | 5 (4) | 7 (6) | 0.101 |
| Cardiovascular | 2 (1) | 10 (8) | 0.001a |
| Othersb | 2 (2) | 4 (3) | 0.260 |
| Comorbidities | n (%) | n (%) | |
|---|---|---|---|
| Smoking | 12 (10) | 3 (4) | 0.513 |
| SAH | 12 (10) | 2 (2) | 0.001a |
| Alcoholism | 12 (10) | 2 (2) | 0.130 |
| DM | 2 (3) | 7 (8) | 0.008a |
| Heart disease | 1 (1) | 7 (9) | 0.000a |
| Lung disease | 1 (1) | 4 (3) | 0.039a |
SAH, systemic arterial hypertension, DM, diabetes mellitus; APACHE II, Acute Physiology and Chronic Health Disease Classification System II; SAPS III, Simplified Acute Physiology Score II.
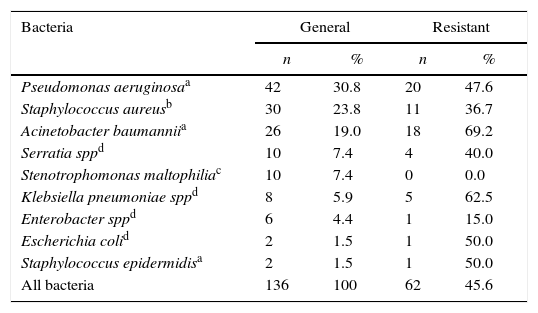
Multi-drug resistant microorganisms were detected in 45.6% of infections, 69.2% of which were caused by A. baumannii, 47.6% by P. aeruginosa, 36.7% by S. aureus, and 42.3% by extended spectrum β-lactamase producing bacteria. No carbapenemase-producing bacteria were observed (Table 2). There was no observed difference in mortality rates among infections caused by resistant or susceptible organisms (27% vs. 46%, p=0.104).
Bacteriological profile of patients diagnosed with ventilator-associated pneumonia who were admitted to the adult intensive care unit of the Hospital de Clinicas of the Federal University of Uberlândia.
| Bacteria | General | Resistant | ||
|---|---|---|---|---|
| n | % | n | % | |
| Pseudomonas aeruginosaa | 42 | 30.8 | 20 | 47.6 |
| Staphylococcus aureusb | 30 | 23.8 | 11 | 36.7 |
| Acinetobacter baumanniia | 26 | 19.0 | 18 | 69.2 |
| Serratia sppd | 10 | 7.4 | 4 | 40.0 |
| Stenotrophomonas maltophiliac | 10 | 7.4 | 0 | 0.0 |
| Klebsiella pneumoniae sppd | 8 | 5.9 | 5 | 62.5 |
| Enterobacter sppd | 6 | 4.4 | 1 | 15.0 |
| Escherichia colid | 2 | 1.5 | 1 | 50.0 |
| Staphylococcus epidermidisa | 2 | 1.5 | 1 | 50.0 |
| All bacteria | 136 | 100 | 62 | 45.6 |
Initial antimicrobial therapy was maintained, escalation, and de-escalation in 57%, 33%, and 10% of cases, respectively. There were no differences in mortality rates among cases in which treatment was de-escalated compared to cases in which it was maintained or escalated (16.6% vs. 33.3%, p=0.160).
The most common error in antimicrobial prescriptions was delay in starting treatment, followed by the interval between doses. Analysis of the influence of prescription errors on mortality rate revealed a 4-fold increase in mortality in patients who received an inappropriate loading dose (p=0.031), and a 3-fold increase when the dosage was not adjusted for renal function (p=0.000) (Table 3).
Evaluation of factors affecting outcomes of patients diagnosed with ventilator-associated pneumonia.
| Discharge | Death | p-Value | |
|---|---|---|---|
| n (%) | n (%) | ||
| Age >60 years | 11(13.4) | 32 (13.4) | 0.000a |
| ICU admission >21 days | 48 (58.5) | 33 (58.5) | 0.106 |
| Prescription errors | n (%) | n (%) | p-Value |
|---|---|---|---|
| Error in loading dose | 1 (1) | 4 (8) | 0.031a |
| Error in maintenance dose | 12 (15) | 11 (22) | 0.304 |
| Error in the interval between doses | 18 (22) | 10 (20) | 0.964 |
| Delay in starting antimicrobial therapy | 57 (70) | 28 (56) | 0.223 |
| Inappropriate adjustment for renal function | 5 (6) | 15 (30) | 0.000a |
| Error in treatment duration | 9 (89) | 4 (8) | 0.299 |
| Conduct | n (%) | n (%) | p-Value |
|---|---|---|---|
| De-escalation | 10 (12) | 2 (4) | 0.160 |
| Continuation | 30 (37) | 12 (24) | 0.685 |
| Maintenance | 42 (51) | 32 (72) | 0.419 |
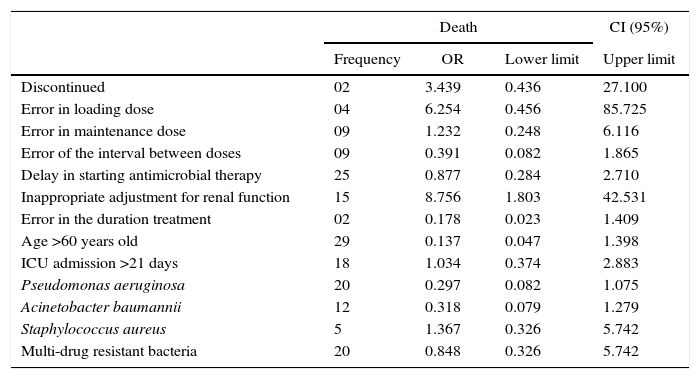
Multiple logistic regression analysis revealed the incorrect adjustment for renal function was the only independent factor associated with increased mortality (Table 4).
Multiple logistic regression analysis of death predictors in ventilator-associated pneumonia in the adult intensive care unit of the Hospital de Clinicas of the Federal University of Uberlândia.
| Death | CI (95%) | |||
|---|---|---|---|---|
| Frequency | OR | Lower limit | Upper limit | |
| Discontinued | 02 | 3.439 | 0.436 | 27.100 |
| Error in loading dose | 04 | 6.254 | 0.456 | 85.725 |
| Error in maintenance dose | 09 | 1.232 | 0.248 | 6.116 |
| Error of the interval between doses | 09 | 0.391 | 0.082 | 1.865 |
| Delay in starting antimicrobial therapy | 25 | 0.877 | 0.284 | 2.710 |
| Inappropriate adjustment for renal function | 15 | 8.756 | 1.803 | 42.531 |
| Error in the duration treatment | 02 | 0.178 | 0.023 | 1.409 |
| Age >60 years old | 29 | 0.137 | 0.047 | 1.398 |
| ICU admission >21 days | 18 | 1.034 | 0.374 | 2.883 |
| Pseudomonas aeruginosa | 20 | 0.297 | 0.082 | 1.075 |
| Acinetobacter baumannii | 12 | 0.318 | 0.079 | 1.279 |
| Staphylococcus aureus | 5 | 1.367 | 0.326 | 5.742 |
| Multi-drug resistant bacteria | 20 | 0.848 | 0.326 | 5.742 |
R2 of 0.674; OR=odds ratio; CI=confidence interval.
Although guidelines for VAP treatment are available, it remains the most prevalent infection in the ICU and is associated with high mortality rates.3 The high mortality rate in patients with VAP in this study (35%) is similar to rates of 32.1%1 and 44.3%11 reported in other Brazilian investigations, as well as a review study in which the rate varied from 14% to 70%.12–15
The higher mortality rates in older patients were likely due to impaired functional status with advancing age. This observation was supported by a study in which age over 55 years was an independent predictor of mortality in patients with VAP (p=0.005).11 Chronic diseases such as diabetes mellitus as well as heart and lung disease were associated with poor prognosis of patients with VAP. This finding also reported by Resende et al., in which the presence of comorbidities was significantly associated with mortality (p=0.029).16
The predominance in this study of Gram-negative bacteria, including P. aeruginosa and A. baumannii, is similar to reports from other countries in South America,17 the United States,18 and Turkey.19 VAP is often related to high rates of resistant bacteria.20 The incidence of multi-drug resistant Acinetobacter and Pseudomonas is increasing and had been associated with increased ICU stays, mechanical ventilation, and possibility of inappropriate treatment in patients receiving standard therapy.5,18,19,21
The lack of association between bacterial resistance and mortality has also been described in the literature22 and can be explained by differences between study populations, preexisting comorbidities, infection severity, and rate of inappropriate empirical treatment. Several studies have demonstrated that the association between mortality and antimicrobial resistance differed from our sample with respect to age, as we included older patients, compared to an average age of 63.4 years23 and 62.3 years,24 and with higher rate of comorbidities. Heart disease and lung diseases were reported in 25% and 20% of patients, respectively.24 These findings reinforce the association between increasing impairment of functional status with age, and the presence of chronic diseases.
The Surviving Sepsis Campaign emphasizes the importance of daily reevaluation of antimicrobial therapy based on the results of culture proliferation assays with the aim of discontinuing treatment, when possible, to reduce antimicrobial resistance, toxicity, and costs.10 High rate of antibiotic maintenance (57% of maintenance vs 43% de-escalation or escalation) has been described in the literature.6 Rello et al.4 considered the low percentage of therapy de-escalation to be due to the high rate of infection caused by multi-drug resistant bacteria including non-glucose fermenting strains (P. aeruginosa and A. baumannii) that were also prevalent in our sample population. Although intended to reduce possible antimicrobial resistance, toxicity, and costs, treatment de-escalation is less likely for infections caused by drug-resistant infections, as also described by Alvarez-Lerma et al.25 They reported that reduction of the initial spectrum of antibiotics occurred only in 23% of patients infected with resistant pathogens compared to 68% of patients infected with sensitive microorganisms (p<0.001).
A multicenter study conducted in the United States,6 as well as investigations carried out in Spain4 and Greece,26 found that mortality rates were significantly reduced after de-escalation of antibiotic treatment. However, subsequent investigations, including our study, did not find any correlation between treatment de-escalation and patient mortality.25 The different results after evaluating the influence of therapy de-escalation on mortality were possibly due to confounding factors. Among these is the difficulty in distinguishing the influence on mortality rates due to treatment de-escalation itself or administration an appropriate therapy, since a correlation between appropriate therapy and higher de-escalation rates has been observed.27 In one study, Giantsou et al.26 included only patients receiving appropriate therapy and observed significantly lower mortality rates after therapy de-escalation compared to maintenance. Differences in antimicrobial susceptibility may also explain different mortality rates, as observed in a multicenter study that found significantly lower mortality rates when therapy was discontinued (p=0.001). However, the rate of infection by resistant bacteria was much lower than in our study, which might have influenced the differences in observed results.6
Error in starting antibiotic administration, the most frequently detected error in our study, probably occurred due to a lack of communication between multidisciplinary teams to immediately initiate the antibiotic as soon as VAP was diagnosed. The complex system of drug prescription also included other circumstances that contribute to errors, such as lack of attention, excessive workload, lack of communication between teams, and lack of knowledge and training of prescribing physicians. Errors in prescribing antimicrobial agents cause short- and long-term consequences that are not just restricted to individuals: they can lead not only to inadequate clinical response and increased morbidity and mortality, but also involve the community by contributing to increased bacterial resistance.28 The lack of increased mortality in patients with delayed start of antibiotic treatment in this study disagreed with other reports emphasizing the relationship between early administration of antibiotics and reduced mortality, as reported by Levy et al.,29 in which the administration of antibiotics within the first hour after diagnosis of severe sepsis and septic shock reduced the mortality rate from 37% to 30.8% (p=0.001).
In this study, prescription of inappropriate antimicrobial loading doses and not adjusting dosage for renal function were determinant factors related to increased mortality. The 4-fold increased mortality (p=0.031) observed in patients with inappropriate loading dose was probably due to an inability to reach proper antimicrobial concentrations at the target site. The lack of knowledge and attention in the initial administration of higher doses or at shorter intervals were determinant for the development of unfavorable outcomes among these patients.
Although renal function was evaluated daily in the adult ICU of the Federal University of Uberlândia, a significant number of errors in adjusting for renal function caused a 3-fold increase in mortality rate (p=0.000). In addition, incorrect adjustment for renal function was the only independent factor associated with mortality in the multiple logistic regression analysis. This error was probably due to a lack of attention to adjust for current creatinine clearance, ease of copying electronic prescriptions from the previous day, and negligence in prescribing an extra dose after hemodialysis. The negative influence on the outcome of these patients was due to the deleterious effects induced by toxic levels of antimicrobial agents when the indicated dose was not reduced, or not to reach the appropriate therapeutic level when the extra dose after hemodialysis was not recommended. These factors were described by Carneiro et al.,30 who reported a very high rate (43.7%) of inappropriate adjustment for renal function.
Prescription errors influenced the mortality rates of patients with VAP, underscoring the challenge of proper VAP treatment, which requires continuous reevaluation to ensure that clinical response to therapy meets expectations.
The limitation of this study is due to retrospective design, since the data were obtained from information abstracted from medical records.
ConclusionsIn conclusion, this study observed that de-escalation of antibiotic therapy and VAP due to resistant bacteria did not influence mortality rates. Inappropriate loading dose and lack of adjustment for renal function were more frequent in patients who died. Multiple logistic regression analysis revealed the incorrect adjustment for renal function was the only independent factor associated with increased mortality.
Conflicts of interestThe authors declare no conflicts of interest.