Ventilator-associated pneumonia (VAP) is one of the most common healthcare-associated infections (HAI) in neonates admitted in neonatal intensive care units (NICUs).
MethodsWe did a systematic review using PRISMA methodology to identify the main etiological agents in Brazilian NICUs. Eligible studies published without period restriction were identified in PUBMED, SCIELO, LILACS and DOAJ. Studies were included if they were conducted in neonates hospitalized at NICU. Studies done in outpatient care, neonates outside NICU, emergency department, primary care, long-term care facilities or a combination of these were excluded.
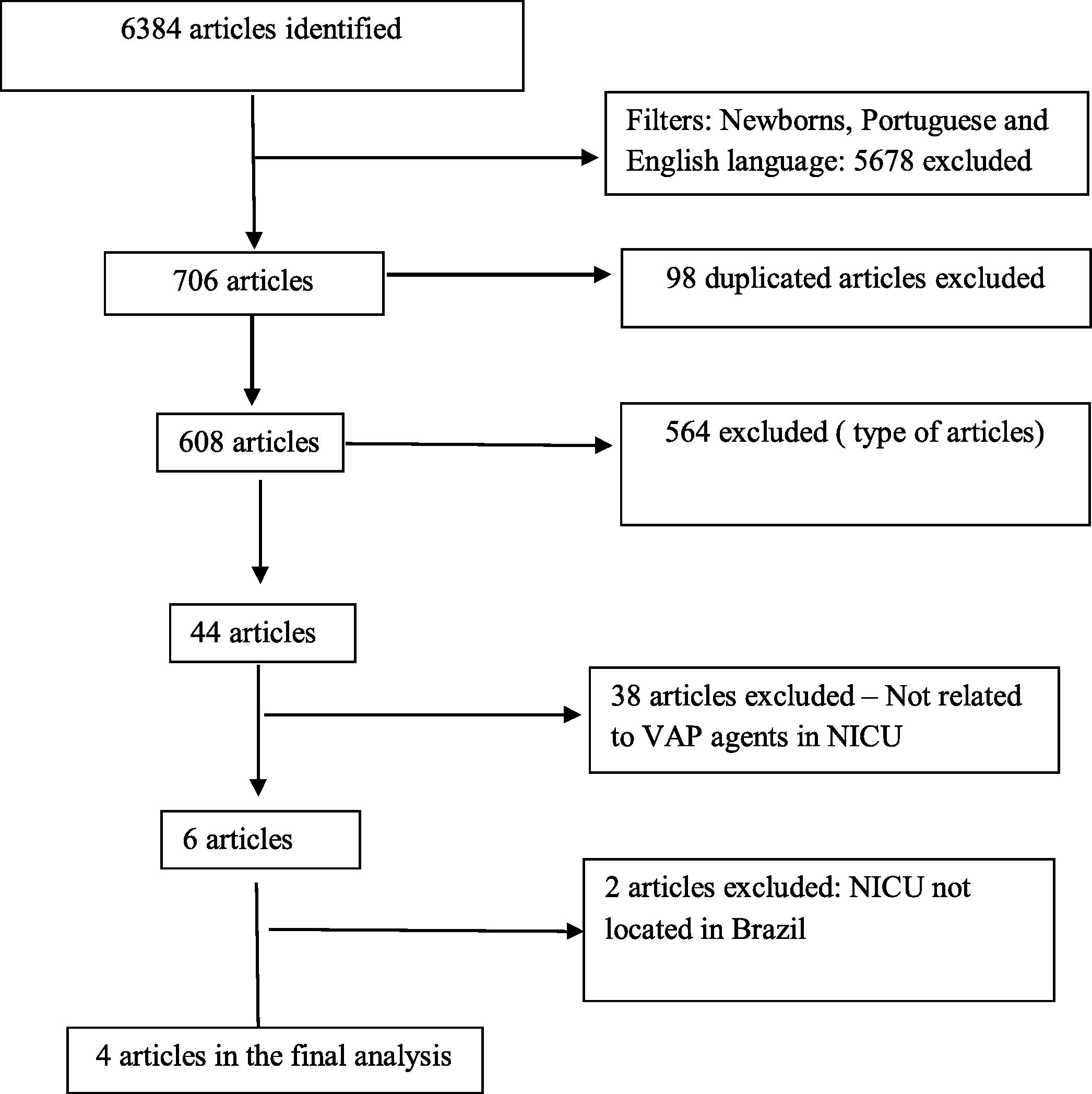
ResultsWe identified 6384 articles in the initial search and four papers met the inclusion criteria. In all studies included, rates of device-associated infections were described, including VAP rates. The VAP incidence density, in exclusively Brazilian NICU, ranged from 3.2 to 9.2 per 1000 ventilator-days. Pneumonia was described as the main HAI in NICU in one article, as the second type of HAI in two other articles and as the fourth type of HAI in the last one. The main pathogens causing all HAI types were described in three of four articles, but, none of the articles reported which pathogens were related or associated to VAP.
ConclusionEtiological agents causing VAP in Brazilian NICUs are, until the present time, not known.
Surveillance, prevention and control of healthcare-associated infections (HAI) in intensive care units, including pediatric intensive care units (PICU) and neonatal intensive care units (NICU) are a global concern, mainly due to high prevalence of multi-drug resistant bacteria in many of these units.1 In 2017, World Health Organization (WHO) published a list of antibiotic-resistant ``priority pathogens’’. The most critical group of all includes Acinetobacter, Pseudomonas and various Enterobacteriaceae (including Klebsiella, E. coli, Serratia, and expanded), carbapenem-resistant or extended-spectrum beta-lactamase (ESBL) producers. They are frequently related to severe bloodstream and pneumonia infections in intensive care units.2
Pneumonia is one of the most common HAI in neonates which is diagnosed using a combination of imaging, clinical and laboratory criteria.3 Ventilator-associated pneumonia (VAP) occurs when the patient is on mechanical ventilation for more than two calendar days on the date of diagnosis and the ventilator was in place on the date of event or the day before.4 VAP accounts for up to 32.2 % of HAI among neonates.5
A recent meta-analysis of observational studies identified 10 variables as independent risk factors for the development of VAP, including length of stay in NICU (OR 23.45), reintubation (OR 9.18), enteral feeding (OR 5.59), mechanical ventilation (OR 4.04), transfusion (OR 3.32), low birth weight (OR 3.16), premature infants (OR 2.66), parenteral nutrition (OR 2.30), bronchopulmonary dysplasia (OR 2.21), and tracheal intubation (OR 1.12).6
Several surveillance systems VAP rates in neonates around the world are NEO-KISS (Nosocomial infection surveillance system for preterm infants on neonatology departments and ICUs) in Germany, neonIN Surveillance Network in UK, and National Healthcare Safety Network (NHSN) in USA.7–9
In a recent report of a national electronic surveillance of VAP rates in neonates, covering 376 hospitals from all Brazilian regions, the incidence density was found to be 7.7, 8.4, 7.5, 7.8, and 8.1 for neonates <750 g, 751–1000 g, 1000–1500 g, 1501–2500 g, and >2500 g, respectively. Despite these important data, no information was available concerning the etiology of VAP in neonates.10 VAP rates vary in different regions of Brazil. In Rio de Janeiro state, the reported VAP incidence density in 2016 was 5.7 cases per 1000 ventilator days in neonates born with more than 2500 g, with no description of etiological agents.11
Knowledge about VAP rates in neonates and the respective causal agents is critical to define which strategies should be prioritized by infection control committees to reduce morbidity and mortality.
The aim of this systematic review was to identify studies reporting the etiological agents causing VAP, in Brazilian NICU.
Materials and methodsThis systematic review was conducted according to recommendations of the PRISMA guidelines for reporting systematic reviews.12
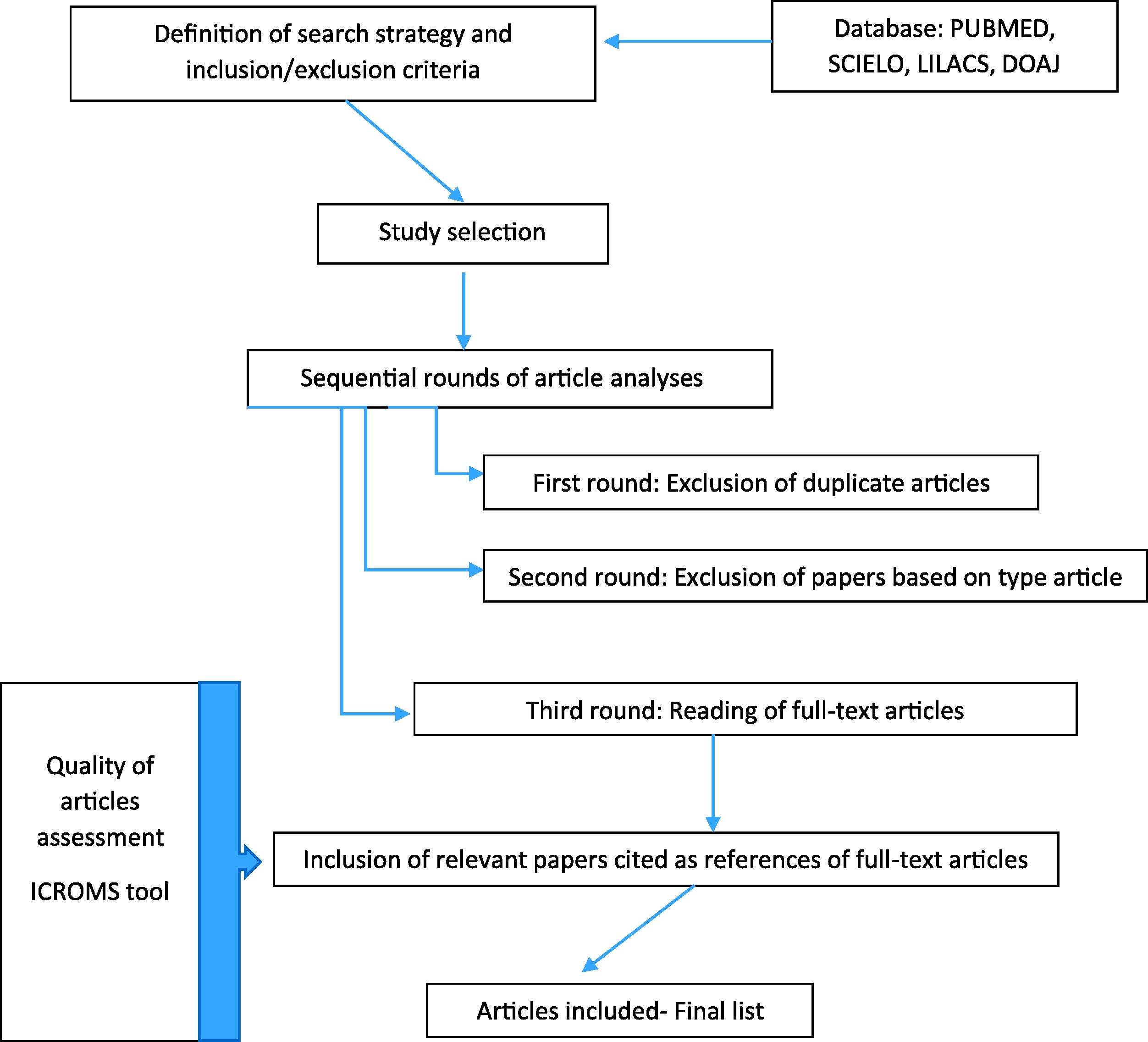
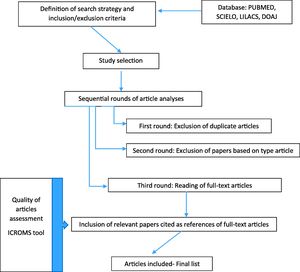
Search strategyThe search was carried out for publications in PUBMED, SCIELO, LILACS and DOAJ using the search term: ``ventilator associated pneumonia’’, without period restriction, limiting results by age (newborns) and English and Portuguese languages (Fig. 1).
Eligibility criteriaInclusion criteriaStudies were eligible for full-text review if they were conducted in hospitalized newborns in NICU setting and reported the etiological agents. Study designs included review studies, multicenter studies, cohort studies, case series, and retrospective studies.
Exclusion criteriaLetters, notes, conference abstracts, and opinion articles were excluded. Studies conducted in outpatient care, newborns outside NICU setting, emergency department, primary care, long-term care facilities, or a combination of the above were also excluded.
Study selectionThe search was conducted independently by five investigators (ARAS, RMBV, RSJ, GJTB, and TCS). The differences in opinion regarding any inclusion criteria for article selection were resolved in a weekly group discussion. After applying the search criteria and filters to each database, we conducted three rounds of article analyses before selecting the final list of publications for inclusion:
- a)
First-round: Exclusion of duplicate articles.
- b)
Second-round: Exclusion of papers based on type of article.
- c)
Third round: Reading of the full-text articles.
- a)
After the third round, relevant papers cited as references of full-text articles were included for analysis, if they fulfilled the eligibility criteria.
Data collectionData were extracted using a standardized data-extraction form which summarized the study details including authors, year of publication, place where the study was conducted, and time frame of the study.
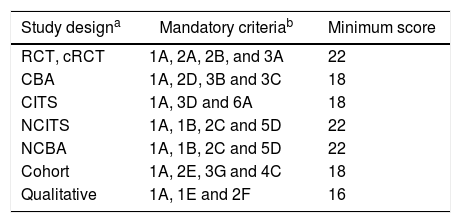
Quality of articles and risk of biasQuality of articles was assessed using the integrated quality criteria for systematic review of multiple study designs (ICROMS) tool.13 In this methodology, it is possible to analyze and integrate studies of different designs using the following criteria. In summary, the tool consists of two parts: the first is a list of quality criteria specific for each study design, as well as criteria applicable across all study designs by using a scoring system and the second is a ‘decision matrix’, which specifies the robustness of the study by identifying minimum requirements according to the study type and the relevance of the study to the review question. Only studies with minimum scores and mandatory criteria, according the ICROMS methodology were included in the final analysis (Annex). For cohort studies, a minimum score of 18 points was necessary to be included in the review.
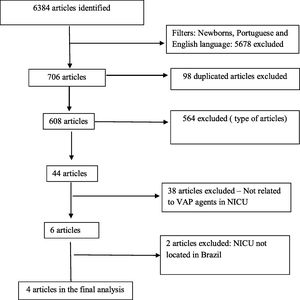
ResultsAccording to the systematic review criteria we identified 6384 articles in the initial search and just four papers met the inclusion criteria for the final analysis (Fig. 2).
All four reports included were cohort studies, and two of them were multicenter studies (one including NICUs from Brazil and other countries, and one including only Brazilian NICUs). In all studies, device-associated infection rates were described, including VAP rates. VAP incidence density in the studies ranged from 2.4 to 13.2 per 1000 ventilator-days in all NICUs and from 3.2 to 9.2 per 1000 ventilator-days in studies that included only Brazilian NICUs
Pneumonia was described as the main HAI in NICU in one article, the second type of HAI in two other studies, and the fourth type of HAI in the last study.
The main pathogens causing the HAI were described in three of the four articles, but, none reported which pathogens were related or associated to VAP.
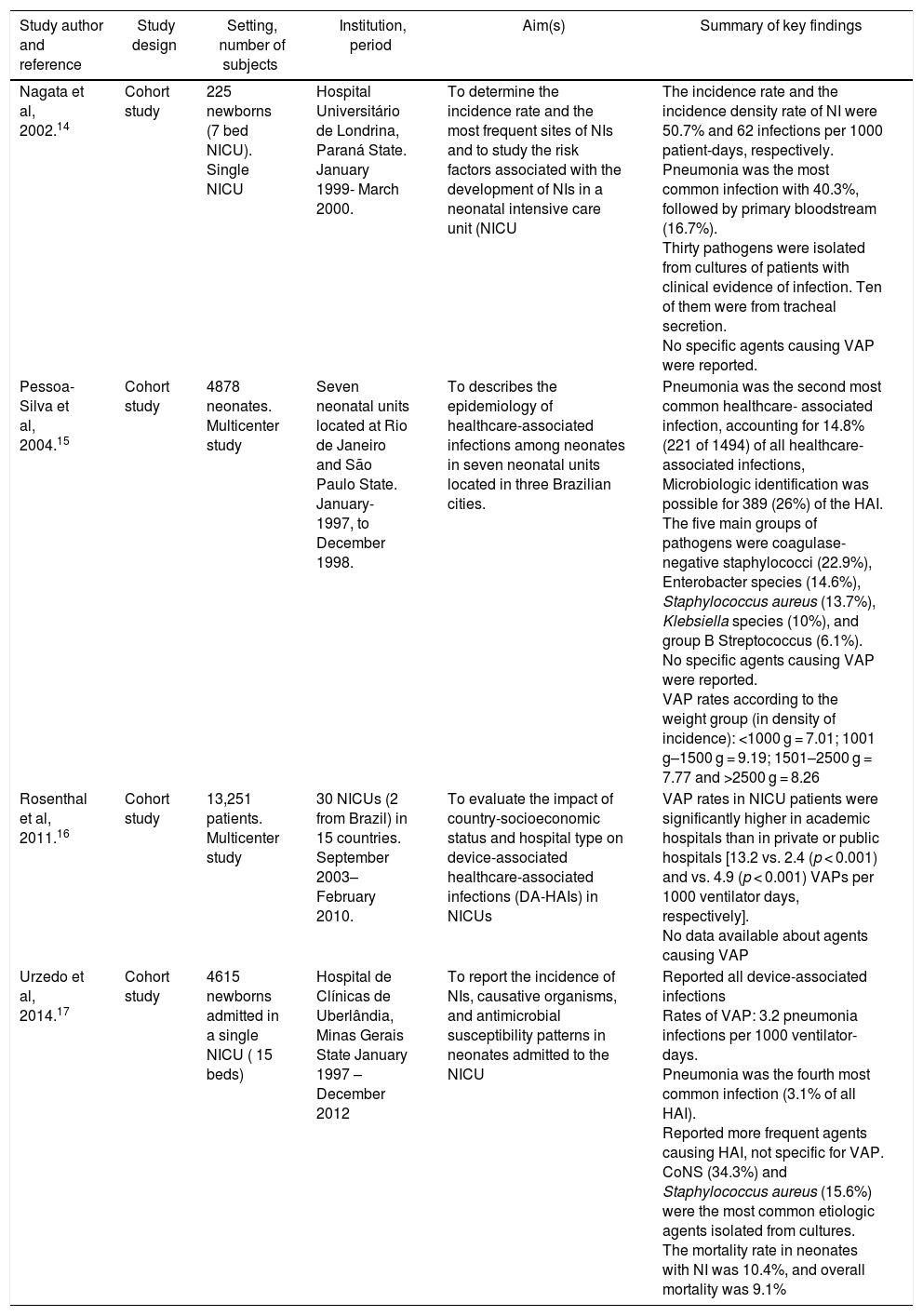
The study design, setting, number of subjects, country, study period, aim, interventions applied, and summary of key findings design of articles included are shown in Table 1. All studies included reached at least 18 points according ICROMS methodology.
Reports of VAP studies in Brazilian NICU – systematic review.
| Study author and reference | Study design | Setting, number of subjects | Institution, period | Aim(s) | Summary of key findings |
|---|---|---|---|---|---|
| Nagata et al, 2002.14 | Cohort study | 225 newborns (7 bed NICU). Single NICU | Hospital Universitário de Londrina, Paraná State. January 1999- March 2000. | To determine the incidence rate and the most frequent sites of NIs and to study the risk factors associated with the development of NIs in a neonatal intensive care unit (NICU | The incidence rate and the incidence density rate of NI were 50.7% and 62 infections per 1000 patient-days, respectively. Pneumonia was the most common infection with 40.3%, followed by primary bloodstream (16.7%). Thirty pathogens were isolated from cultures of patients with clinical evidence of infection. Ten of them were from tracheal secretion. No specific agents causing VAP were reported. |
| Pessoa-Silva et al, 2004.15 | Cohort study | 4878 neonates. Multicenter study | Seven neonatal units located at Rio de Janeiro and São Paulo State. January-1997, to December 1998. | To describes the epidemiology of healthcare-associated infections among neonates in seven neonatal units located in three Brazilian cities. | Pneumonia was the second most common healthcare- associated infection, accounting for 14.8% (221 of 1494) of all healthcare-associated infections, Microbiologic identification was possible for 389 (26%) of the HAI. The five main groups of pathogens were coagulase-negative staphylococci (22.9%), Enterobacter species (14.6%), Staphylococcus aureus (13.7%), Klebsiella species (10%), and group B Streptococcus (6.1%). No specific agents causing VAP were reported. VAP rates according to the weight group (in density of incidence): <1000 g = 7.01; 1001 g–1500 g = 9.19; 1501–2500 g = 7.77 and >2500 g = 8.26 |
| Rosenthal et al, 2011.16 | Cohort study | 13,251 patients. Multicenter study | 30 NICUs (2 from Brazil) in 15 countries. September 2003–February 2010. | To evaluate the impact of country-socioeconomic status and hospital type on device-associated healthcare-associated infections (DA-HAIs) in NICUs | VAP rates in NICU patients were significantly higher in academic hospitals than in private or public hospitals [13.2 vs. 2.4 (p < 0.001) and vs. 4.9 (p < 0.001) VAPs per 1000 ventilator days, respectively]. No data available about agents causing VAP |
| Urzedo et al, 2014.17 | Cohort study | 4615 newborns admitted in a single NICU ( 15 beds) | Hospital de Clínicas de Uberlândia, Minas Gerais State January 1997 – December 2012 | To report the incidence of NIs, causative organisms, and antimicrobial susceptibility patterns in neonates admitted to the NICU | Reported all device-associated infections Rates of VAP: 3.2 pneumonia infections per 1000 ventilator-days. Pneumonia was the fourth most common infection (3.1% of all HAI). Reported more frequent agents causing HAI, not specific for VAP. CoNS (34.3%) and Staphylococcus aureus (15.6%) were the most common etiologic agents isolated from cultures. The mortality rate in neonates with NI was 10.4%, and overall mortality was 9.1% |
This review highlighted the absence of data on the causative agents related to VAP in Brazilian NICU. VAP is one of most prevalent infections within NICU in many countries. For example, Tan et al. studied the epidemiology of neonatal VAP in China. In an analysis of 16,587 newborns, the incidence and case fatality rates were 42.8% and 16.4%, respectively. Gram-negative bacteria were detected in 77.6% of cultures, followed by Gram-positive bacteria (18.8%) and fungi (3.7%). Gram-negative bacteria were resistant to meropenem, imipenem, and ciprofloxacin in rates of 1.5–25.0%, 4.9–29.0%, and 8.5–24.7%, respectively. Gram-positive bacteria have resistance rates as high as 80.3–91.9% to oxacillin.6 Other study conducted in 304 NICU of USA analyzed device-associated infections, including VAP. Pooled mean incidence rates of VAP by birth weight category (750 g or less, 751–1000 g, 1001–1500 g, 1501–2500 g, and more than 2500 g) were 2.36, 2.08, 1.28, 0.86, and 0.72, respectively. The frequencies of isolated pathogens were 16% of Pseudomonas species, 15% S. aureus, and 14% Klebsiella species.18 New methods for rapid detection of pathogens related to VAP (Unyvero multiplex PCR) could provide additional information for clinical decision making, especially in neonates and in the setting of nosocomial pneumonia, also contributing to reduce inappropriate antimicrobial therapy.19
In our review, just one study specified the agents causing HAI, but the description referred to all infections, failing to single out the etiology of VAP. Coagulase-negative staphylococci was the main pathogen identified, possibly related to bloodstream infections rather than VAP.15
Despite these relevant epidemiological data included in final analysis, no studies carried out in Brazilian NICU reporting the pathogens related to VAP could be identified. Probably, the etiologic agents are similar to those reported in Latin America, but the resistance profile could be different in each country.20 This information is mandatory to guide governmental policies and regional and local actions that should be implemented to prevent VAP in neonates. To our knowledge, this is the first systematic review in Brazil studying agents causing VAP in NICU.
Data of VAP rates in, exclusively, Brazilian NICU were described in two reports, with incidence density ranging from 3.2 to 9.2/1000 ventilator-days. These data are higher than those reported for NHSN surveillance, but similar to rates reported by Wójkowska-Mach et al. in six Polish NICU (3.1/1000 NICU patient days) between 2009 and 2011 and by Tekin et al. in a 4-year device-associated nosocomial infections surveillance in a single NICU of Turkey (6.4 per 1000/ventilator days).21,22 In another study included in our analysis, Rosenthal et al described VAP rates in 30 NICU from a multicenter study and included two Brazilian NICU, but it was not possible to determine the exact rate in these two units.
There are some limitations in our review. Studies reporting VAP etiological agents that could have been reported in others sources of research such as congress abstracts and regional governmental reports, were not included in this research. Usually these reports come from single healthcare institutions but provide useful information about local data.
In conclusion, etiological agents causing VAP in Brazilian NICU are, until the present time, not known. We suggest inclusion of VAP etiological agents in systematic reports of Brazilian National system surveillance as well more studies including epidemiology data from all Brazilian regions.
FundingThe author(s) received no financial support for the research, authorship, and/or publication of this article.
Peer review statementNot commissioned; blind peer-reviewed.
Conflicts of interestThe authors declare no conflicts of interest.
The authors thank all members of Laboratory of Teaching of Prevention and Control of Healthcare-Associated Infections (Cristiane Henriques Teixeira, Lucia Santos Werneck, Andreia Medeiros and Patricia Ribeiro) for support.
Annex. Quality criteria for application per study design. Integrated quality criteria for review of multiple study designs (ICROMS)
| Quality criteria | Study designb | |||||||
|---|---|---|---|---|---|---|---|---|
| Dimension | Specific criteriaa | RCT | CBA | CITS | NCITS | NCBA | CS | QUAL |
|
| ++ x x x x | ++ x x x x | ++ + x x x | ++ ++ + x x | ++ ++ + x x | ++ x x x x | ++ x x + ++ |
|
| ++ ++ x x x x | x x x ++ x x | x x x x x x | x x ++ x x x | x x ++ x x x | x x x x ++ x | x x x x x ++ |
|
| ++ x x x + + x | x ++ ++ x + + x | x x x ++ + + x | x x x x + + x | x x x x + + x | x x x x + + ++ | x x x x x + x |
|
| + + + | x x + | x x + | x x + | x x + | x x ++ | x x + |
|
| + x x x | + x x x | + x x x | + x x ++ | + x x ++ | x + x x | x x + x |
|
| x x + | x x + | ++ + + | x x + | x x + | x x + | x x + |
|
| + + + + + | + + + + + | + + + + + | + + + + + | + + + + + | + + + + + | + + + + + |
a Applicability of quality criteria to each study design: + Criteria to be included in quality assessment for study design; ++ Mandatory criteria to be met for quality assessment; x Criteria not to be applied for quality assessment of study design.
b Study designs: RCT = randomized controlled trial; CBA = controlled before-after; CITS ¼ controlled interrupted time series; CS = cohort study; NCITS = non-controlled interrupted time series; NCBA = non-controlled before-after; QUAL = qualitative.
a Study Designs: RCT = randomized controlled trial; CBA = controlled before-after; CITS = controlled interrupted time series; cRCT = cluster-randomized controlled trial; NCITS = noncontrolled interrupted time series; NCBA = non-controlled before-after.
b Scores applicable to each criterium: Yes (criterion met) = 2 points; Unclear (unclear whether or not the criterion is met) = 1 point; No (criterion not met) = 0 points.
Adapted from Zingg W et al. Innovative tools for quality assessment: integrated quality criteria for review of multiple study designs (ICROMS). Public Health 2016;133:19–37.