Technologies and life support management have enhanced the survival of preterm infants. The immune system of newborns is immature, which contributes to the occurrence of healthcare-associated infections. The overlap of several conditions with neonatal sepsis and the difficulty of diagnosis and laboratory confirmation during this period result in a tendency to over-treat neonatal sepsis. The use of antimicrobial agents is a risk factor for multidrug-resistant bacterial infections. This work aimed to perform a systematic review of the relationship between inadequate use of antimicrobial agents and increase in neonatal sepsis related to healthcare assistance, due to bacterial resistance.
MethodsOur population, exposition, comparison, outcome and study type was as follows: P: hospitalized neonates with sepsis diagnosis, E: inappropriate use of antimicrobial agents, C: adequate use of antimicrobial agents or no indication of infection, O: resistant bacterial infection, and S: original studies. We performed searches in the PubMed, Scopus, Virtual Health Library (Scielo, LILACS, and MEDLINE), and Embase without limits on time, language, and the references of the articles found. Fourteen studies were included and assessed using the Grading of Recommendations, Assessment, Development, and Evaluation, Newcastle, and the Strengthening the Reporting of Observacional Studies in Epidemiology methodologies.
ResultsAll studies found were observational and started with a low-quality evidence level in the Grading of Recommendations, Assessment, Development, and Evaluation.
ConclusionsDespite their low-quality evidence, the studies demonstrated the association between inadequate use of antimicrobial agents and increase of neonatal resistant bacterial healthcare-associated infections in neonatal units. However, there is significant difficulty in conducting high-quality studies in this population due to ethical issues tied to randomized trials. Therefore, new studies should be encouraged to recommend adequate treatment of newborns without increasing the risk of healthcare-associated infections by multidrug-resistant bacteria.
In recent years, the improvement of technologies for advanced life support applied to prematurity complications resulted in increased survival of preterm infants with earlier gestational age and low birth weight.1,2 Due to immaturity of the immune system and the need for several invasive procedures, hospitalized neonates, especially premature infants, are susceptible to healthcare-associated infections (HAI). This is one of the most significant adverse events associated with morbidity and mortality in this age group.1–5
The diagnosis of HAI in newborns is difficult due to the lack of specific symptoms. At this age, HAI commonly shares symptoms with other diseases, such as respiratory distress syndrome, prematurity hypotension, metabolic disorders, and others.1,6–10 Beginning early treatment is fundamental for improving the prognosis of these patients. Therefore, treatment is initiated before receiving laboratory results, which contributes to the exposure of this population to antimicrobial agents (ATM), often unnecessary.6–11 Furthermore, the difficulty in obtaining sufficient samples for cultures and the low sensitivity of this test leads many physicians to decide to continue ATM for treating presumed neonatal sepsis,6,8 which is up to eight-fold over-treated than confirmed HAI cases, according to some studies.6
The increase of HAI caused by resistant bacteria is a multifactorial issue. Currently, it is a worldwide concern due to the associated high morbidity and mortality and increased hospitals costs.1,3,8,12 The inability of the pharmaceutical industry to create new drugs at the same rate as the development of resistance is also an important issue to consider.3,9–11 The excessive use of ATM, especially broad-spectrum agents, is already recognized as a key factor for the development of resistance.1,6,7,9,11–16 In recent years, the emergence of increasingly resistant strains has been observed,2,6,8 as demonstrated by clinical trials in adult and pediatric populations. However, in the neonatal population, there is a lack of studies assessing this association.8,13,17
The aim of this work was to perform a systematic review of the relationship between inadequate use of ATM and increase in neonatal sepsis related to healthcare assistance, due to bacterial resistance. For this review, the attributes considered when selecting the articles were: population, exposition, comparison, outcome, and study type, according to the acronym PECOS.18,19 The definition was as follows: P, neonates with sepsis diagnosis in Neonatal Units; E: inappropriate use of ATM; C, adequate use of ATM or no indication of infection; O, resistant bacterial infection; S, original studies.
MethodsLiterature reviewThe articles were selected using the international guideline outlined by PRISMA, which coordinates the process of performing meta-analyses and systematic reviews.18
The search was carried out without limits on language or date of the study, using the PubMed, Scopus, Virtual Health Library (Scielo, LILACs, and MEDLINE) and Embase using the following keywords: “Sepsis,” “Infant, Newborn,” “Anti-Infective Agents,” and “Drug Resistance, Microbial” and their respective Portuguese and Spanish translations up to July 2017. The references from the selected articles were also evaluated and included in the selection if they met the inclusion criteria. PubMed was searched using the MeSH terms: (“Sepsis”[MeSH] AND “Infant, Newborn”[MeSH]) AND “Anti-Infective Agents”[MeSH]) AND “Drug Resistance, Microbial”[MeSH].
Inclusion and exclusion criteriaInclusion criteria: All original articles that included patients with late-onset neonatal sepsis with multi-resistant bacteria, who were treated with broad-spectrum ATM, or with inadequate or prolonged empiric treatment. Additionally, only articles that defined the duration and type of ATM were included.
Exclusion criteria: All articles that included patients with early onset sepsis with multi-resistant bacteria, articles which did not define duration and type of ATM therapy, and articles which considered only risk factors for colonization with multidrug-resistant bacteria.
Some studies that described ATM use but did not comply with the PECOS framework were only used for the discussion section.
Article extraction and data quality evaluationTwo independent reviewers evaluated all titles and abstracts from the database search, with the goal of identifying articles that would generally accomplish the selection criteria.
Full texts of the selected abstracts were acquired and analyzed by the study type, population, intervention, comparison, and outcome to determine their inclusion.
The Grading of Recommendations, Assessment, Development, and Evaluation (GRADE)20–22 approach was applied to evaluate the quality of evidence in the methods and results reported in the studies and summary of findings, as recommended by The Cochrane Collaboration. In the GRADE system,20–22 randomized trials are considered high-quality evidence and observational studies are considered low-quality evidence.
The GRADE score can be reduced due to methodological limitations, inconsistencies, inaccuracies, indirect evidence, and published bias. The quality of evidence can increase due to the magnitude of effect, dose response, and control of all plausible confounding factors.20–22
The Newcastle-Ottawa scale was also used to evaluate methodological limitations in each observational study.21–23
Inconsistency was evaluated based on clinical and methodological heterogeneity. The I2 parameter was not calculated, as a meta-analysis was not performed. Inconsistency was assessed through the use of effect measures and confidence intervals.21–23
Assessments of imprecision used the absolute effect (difference between the exposed and non-exposed groups) and were calculated individually for the studies that provided the effect measure. It was calculated as the Number of patients Needed to Harm (NNH).21–23
The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist24,25 was also used to evaluate the methods and descriptions of observational studies. The evaluation of this information was qualitative.
ResultsThe search of PubMed, Scopus, Embase and the Virtual Health Library resulted in 616 articles. Additionally, the reference analysis resulted in four articles, with a total of 620 articles. Among these, 16 (2.6%) were excluded as duplicates, 587 (94.7%) did not satisfy the inclusion criteria, and two (0.3%) were unclear regarding type and duration of ATM therapy. Of the 15 (2.4%) articles selected, one (0.15%) was excluded because it was only available in Mandarin. The others were evaluated and qualified (Fig. 1).
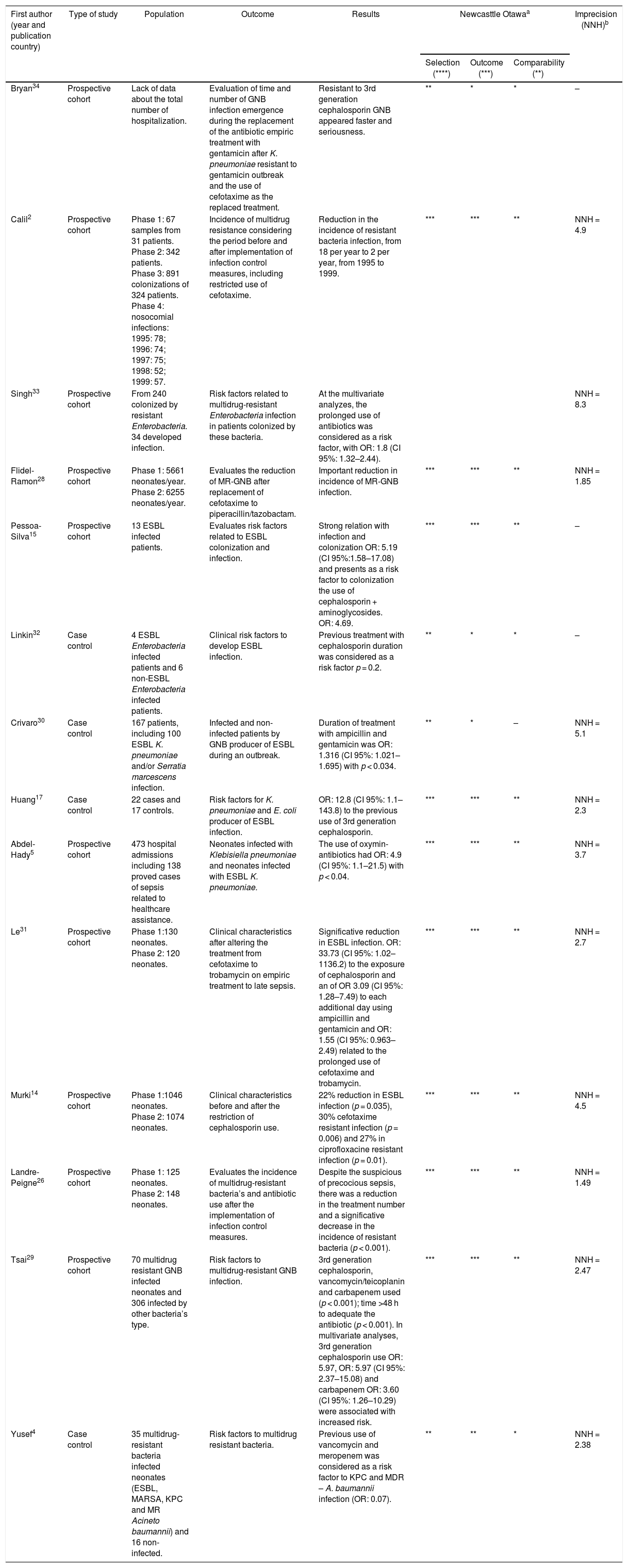
Among the 14 analyzed articles, all of them were observational studies: 10 prospective cohort and four case-control studies. In this review, there were no randomized trials or interventional studies. Ten studies described the appearance of Gram-negative bacteria (GNB) and four studies attributed the use of ATM to resistant bacterial infection and colonization. Further details about the studies are available in Table 1.
Characteristics of studies considering the use of antibiotics associated with increased occurrence of neonatal sepsis related to resistant bacteria.
| First author (year and publication country) | Type of study | Population | Outcome | Results | Newcasttle Otawaa | Imprecision (NNH)b | ||
|---|---|---|---|---|---|---|---|---|
| Selection (****) | Outcome (***) | Comparability (**) | ||||||
| Bryan34 | Prospective cohort | Lack of data about the total number of hospitalization. | Evaluation of time and number of GNB infection emergence during the replacement of the antibiotic empiric treatment with gentamicin after K. pneumoniae resistant to gentamicin outbreak and the use of cefotaxime as the replaced treatment. | Resistant to 3rd generation cephalosporin GNB appeared faster and seriousness. | ** | * | * | – |
| Calil2 | Prospective cohort | Phase 1: 67 samples from 31 patients. Phase 2: 342 patients. Phase 3: 891 colonizations of 324 patients. Phase 4: nosocomial infections: 1995: 78; 1996: 74; 1997: 75; 1998: 52; 1999: 57. | Incidence of multidrug resistance considering the period before and after implementation of infection control measures, including restricted use of cefotaxime. | Reduction in the incidence of resistant bacteria infection, from 18 per year to 2 per year, from 1995 to 1999. | *** | *** | ** | NNH = 4.9 |
| Singh33 | Prospective cohort | From 240 colonized by resistant Enterobacteria. 34 developed infection. | Risk factors related to multidrug-resistant Enterobacteria infection in patients colonized by these bacteria. | At the multivariate analyzes, the prolonged use of antibiotics was considered as a risk factor, with OR: 1.8 (CI 95%: 1.32–2.44). | NNH = 8.3 | |||
| Flidel-Ramon28 | Prospective cohort | Phase 1: 5661 neonates/year. Phase 2: 6255 neonates/year. | Evaluates the reduction of MR-GNB after replacement of cefotaxime to piperacillin/tazobactam. | Important reduction in incidence of MR-GNB infection. | *** | *** | ** | NNH = 1.85 |
| Pessoa-Silva15 | Prospective cohort | 13 ESBL infected patients. | Evaluates risk factors related to ESBL colonization and infection. | Strong relation with infection and colonization OR: 5.19 (CI 95%:1.58–17.08) and presents as a risk factor to colonization the use of cephalosporin + aminoglycosides. OR: 4.69. | *** | *** | ** | – |
| Linkin32 | Case control | 4 ESBL Enterobacteria infected patients and 6 non-ESBL Enterobacteria infected patients. | Clinical risk factors to develop ESBL infection. | Previous treatment with cephalosporin duration was considered as a risk factor p = 0.2. | ** | * | * | – |
| Crivaro30 | Case control | 167 patients, including 100 ESBL K. pneumoniae and/or Serratia marcescens infection. | Infected and non-infected patients by GNB producer of ESBL during an outbreak. | Duration of treatment with ampicillin and gentamicin was OR: 1.316 (CI 95%: 1.021–1.695) with p < 0.034. | ** | * | – | NNH = 5.1 |
| Huang17 | Case control | 22 cases and 17 controls. | Risk factors for K. pneumoniae and E. coli producer of ESBL infection. | OR: 12.8 (CI 95%: 1.1–143.8) to the previous use of 3rd generation cephalosporin. | *** | *** | ** | NNH = 2.3 |
| Abdel-Hady5 | Prospective cohort | 473 hospital admissions including 138 proved cases of sepsis related to healthcare assistance. | Neonates infected with Klebisiella pneumoniae and neonates infected with ESBL K. pneumoniae. | The use of oxymin-antibiotics had OR: 4.9 (CI 95%: 1.1–21.5) with p < 0.04. | *** | *** | ** | NNH = 3.7 |
| Le31 | Prospective cohort | Phase 1:130 neonates. Phase 2: 120 neonates. | Clinical characteristics after altering the treatment from cefotaxime to trobamycin on empiric treatment to late sepsis. | Significative reduction in ESBL infection. OR: 33.73 (CI 95%: 1.02–1136.2) to the exposure of cephalosporin and an of OR 3.09 (CI 95%: 1.28–7.49) to each additional day using ampicillin and gentamicin and OR: 1.55 (CI 95%: 0.963–2.49) related to the prolonged use of cefotaxime and trobamycin. | *** | *** | ** | NNH = 2.7 |
| Murki14 | Prospective cohort | Phase 1:1046 neonates. Phase 2: 1074 neonates. | Clinical characteristics before and after the restriction of cephalosporin use. | 22% reduction in ESBL infection (p = 0.035), 30% cefotaxime resistant infection (p = 0.006) and 27% in ciprofloxacine resistant infection (p = 0.01). | *** | *** | ** | NNH = 4.5 |
| Landre-Peigne26 | Prospective cohort | Phase 1: 125 neonates. Phase 2: 148 neonates. | Evaluates the incidence of multidrug-resistant bacteria’s and antibiotic use after the implementation of infection control measures. | Despite the suspicious of precocious sepsis, there was a reduction in the treatment number and a significative decrease in the incidence of resistant bacteria (p < 0.001). | *** | *** | ** | NNH = 1.49 |
| Tsai29 | Prospective cohort | 70 multidrug resistant GNB infected neonates and 306 infected by other bacteria’s type. | Risk factors to multidrug-resistant GNB infection. | 3rd generation cephalosporin, vancomycin/teicoplanin and carbapenem used (p < 0.001); time >48 h to adequate the antibiotic (p < 0.001). In multivariate analyses, 3rd generation cephalosporin use OR: 5.97, OR: 5.97 (CI 95%: 2.37–15.08) and carbapenem OR: 3.60 (CI 95%: 1.26–10.29) were associated with increased risk. | *** | *** | ** | NNH = 2.47 |
| Yusef4 | Case control | 35 multidrug-resistant bacteria infected neonates (ESBL, MARSA, KPC and MR Acineto baumannii) and 16 non-infected. | Risk factors to multidrug resistant bacteria. | Previous use of vancomycin and meropenem was considered as a risk factor to KPC and MDR – A. baumannii infection (OR: 0.07). | ** | ** | * | NNH = 2.38 |
The symbol * match the score on each item evaluated from the articles.
GNB, Gram-negative bacteria; OR, Odds Ratio; CI, Confidence intervals; MR, multidrug-resistant; ESBL, producers of extended-spectrum β-lactamases; MARSA, Methicillin-resistant Sthaphylococcus aureus; KPC, Klebisiella pneumoniae carbapenemase.
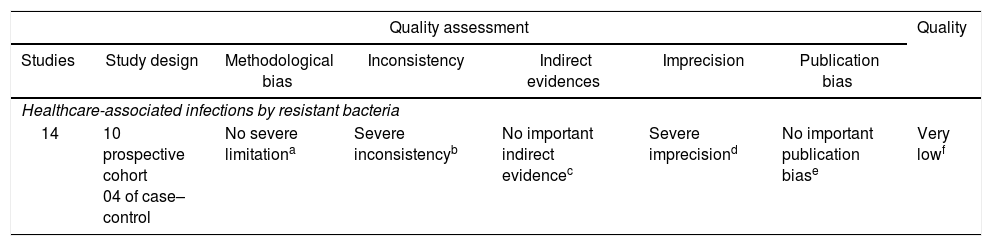
The review considered evidences of very low quality, as determined by the GRADE scale.20 Table 2 shows the GRADE evaluation of all assessed studies.
Summary of studies considering the association of the use of antibiotics and increase occurrence of neonatal sepsis related to resistant bacteria, according to the Grading of Recommendations, Assessment, Development, and Evaluation criteria.
| Quality assessment | Quality | ||||||
|---|---|---|---|---|---|---|---|
| Studies | Study design | Methodological bias | Inconsistency | Indirect evidences | Imprecision | Publication bias | |
| Healthcare-associated infections by resistant bacteria | |||||||
| 14 | 10 prospective cohort 04 of case–control | No severe limitationa | Severe inconsistencyb | No important indirect evidencec | Severe imprecisiond | No important publication biase | Very lowf |
All the studies were observational, which present a greater risk of bias. Although three studies present methodological limitations, nothing was considered as serious to downgrade the score in this item.
Once it was not a meta-analysis, I2 was not calculated. The inconsistency was assessed through measures of effect and confidence interval in seven studies that showed an association between antimicrobial use and increased nosocomial infection by multi-resistant bacteria. One study showed a low inconsistency because the confidence interval crosses the number 1 and other three studies presented very wide confidence intervals.
Although the studies present different methodological differences between themselves and based on PECO question of the review, no serious indirect evidence was observed, since the result and the population are the same in all studies.
The absolute effect (difference between the exposed and non-exposed groups) was considered and calculated for the studies that presented the effect measure. The Number of patients Needed to Harm (NNH) was calculated, which presented important variation, suggesting imprecision of the articles.
In 2002, the Centers for Disease Control (CDC) started a campaign to prevent antimicrobial resistance with 12 steps, including reinforcing the need for caution when choosing an ATM, using ATM for the shortest duration, and restricting ATM use by culture results.2,6,7,9,13,26,27 Despite the fact that the recommendations do not focus specifically on neonatal care, Cantey7 and Patel27 presented the applicability of these guidelines in a neonatal unit.
Calil et al.2 and Landre-Paige et al.26 evaluated the efficacy of the ATM control measures. In a cohort study, Landre-Paige et al.26 demonstrated that simple measures, such as organization of the nursing staff, hand washing, and development of criteria to guide initiation of empiric treatment for early sepsis, were effective in reducing the incidence of nosocomial infections. The empiric treatment used included ampicillin and gentamicin, but was questionable, because in severe cases, the treatment included ceftazidime, which counters the recommendations to reduce the use of cephalosporins.2,10,12,16 The same treatment was maintained during the two phases of the study. Landre-Paige et al. have also shown a significant reduction in the prescription of empiric ATM (from 100% to 51%) as well as a meaningful reduction of HAI (from 10.9 to 2.9/1000 patients per day) and resistant bacteria, mostly GNB (from 79% to 12%).
The prospective cohort study conducted by Calil et al.2 evaluated the impact of periodic training of professionals in a tertiary neonatal unit, including training on the rational use of ATM, hand washing, and the withdraw of ceftriaxone from empiric treatment of early and late sepsis in newborns weighting less than 1500 g. This study demonstrated an important reduction in multidrug-resistant bacteria. Evaluating only colonization by this type of pathogen, they observed an odds ratio (OR) of 2.5 associating the use of ceftriaxone and colonization by multidrug-resistant bacteria, and observing a greater occurrence during ceftriaxone utilization. While assessing the occurrence of infection by a multidrug-resistant microorganism, a decrease from 18 to two infections per year was observed after the implementation of the stewardship program, although the number of nosocomial multidrug-resistant bacterial infections was low.
In a cohort study, Zingg et al.13 also described a reduction of 2.9% per year in the use of ATM, with no increase in mortality. This study observed a low incidence of multidrug-resistant bacterial infections during the study, thus it was not possible to compare data before and after implementation.
In prospective cohort study by Flidel-Rimon et al.28 two periods were evaluated. In the first period, the incidence of infection by resistant Klebsiella pneumoniae was assessed after implementation of hand washing training, improvement of sterile technique, and isolation of patients colonized/infected with resistant bacteria. The first period displayed a notable reduction in the incidence of HAI from 12.5 to 5.3 per 1000 patients, but the incidence of infection by resistant K. pneumoniae remained stable at approximately 2.5 per 1000. However, after modification of the empirical treatment, replacing ceftazidime with piperacillin/tazobactam, the incidence was reduced from 2.5 to 0.45 per 1000 patients.
In a prospective cohort study, Tsai et al.29 compared patients infected by GNB that were sensitive or multidrug-resistant to the first choice ATM. Multivariate analyses revealed that previous use of 3rd generation cephalosporin and meropenem and use of vancomycin and meropenem were risk factors associated with infection by multidrug-resistant bacteria. The empiric treatment guidelines in the neonatal unit permitted the use of vancomycin and cefotaxime, both of which are considered broad-spectrum and 2nd line antibiotics by the CDC.7,27
During the review, eight articles evaluated risk factors of infection by GNB producers of extended-spectrum β-lactamases (ESBL). The incidence of these bacteria is increasing worldwide, which is particularly alarming because this resistance mechanism can be transmitted by plasmids, which facilitate the acquisition of resistance by other bacteria.5,14,15,17,30–33
In searching for risk factors of infection by resistant GNB, the cohort studies conducted by Abdel-Hady et al.,5 Le et al.,31 and Murki et al.14 and the case-control study conducted by Huang et al.17 identified the previous use of 3rd and 4th generation cephalosporins as risk factors for infections by ESBL-producing GNB. Abdel-Hady et al.5 observed an OR of 4.9 in the association with previous use of 3rd generation cephalosporins. In a study by Le et al.,31 previous use of cephalosporins presented an OR of 33.7, and an OR of 3.09 for each additional day of ampicillin and gentamicin use and an OR of 1.55 associated with prolonged exposure to cefotaxime and tobramycin. Huang et al.17 demonstrated an OR of 12.8 for the association with the previous use of 3rd generation cephalosporins, and Murki et al.14 reported a significant statistical association between previous use of cephalosporins and infection by ESBL-producing GNB.
In a cohort study, Singh et al.33 evaluated patients colonized by ESBL-producing GNB who developed infection by this bacteria. A multivariate analysis identified birth weight greater than 1000 g and prolonged exposition to ATM, independent of the ATM class, as risk factors. In case-control studies, Crivaro et al.30 and Linkin et al.32 also identified prolonged ATM exposure as a risk factor associated with colonization and infection by ESBL-producing GNB. These two studies evaluated infection associated with previous colonization and had a small sample of multidrug-resistant infections, increasing the risk of study bias. The study by Crivaro et al.23 presented an adequate sample and better methodological quality for evaluating colonization. However, the study by Linkin et al.29 was based on a small sample size and a literature review of risk factors for colonization and infection by ESBL-producing bacteria.
Pessoa-Silva et al.15 conducted a cohort study to identify risk factors for infection by ESBL-producing bacteria. The authors did not identify previous use of ATM as a risk factor, although the colonization by ESBL-producing bacteria was a risk factor, with an OR of 5.9. In the same study, the authors found that previous use of 3rd generation cephalosporins and aminoglycosides were associated with infection by ESBL-producing GNB, with an OR of 4.6 during the first nine days of hospitalization and an OR of 2.43 after nine days. The authors concluded that previous use of 3rd generation cephalosporins and aminoglycosides are also risk factors for infection by ESBL-producing bacteria.
The oldest publication identified in the present review was a cohort study from the early 1980s conducted by Bryan et al.34 The study included the replacement of gentamicin by cefotaxime following an outbreak of gentamicin-resistant K. pneumoniae in a neonatal unit. For 11 years, before the appearance of these resistant bacteria, the first choice of empirical treatment was gentamicin. After eight months of cefotaxime use, no resistant K. pneumoniae was identified. However, cefotaxime-resistant Enterobacter cloacae emerged as an important pathogen and continued to be prevalent even after the restored use of gentamicin as empiric treatment. Despite the fact that cefotaxime was associated with faster development of resistance, the mortality rate did not increase during the study. This study has several methodological limitations, including a small sample size and lack of statistical tests in their analyses. The study conclusion can be explained by the fact that cefotaxime was a new drug at that time, with the same spectrum to GNB, but with fewer nephrotoxic and ototoxic effects than gentamicin. Spritzer et al.35 presented a similar conclusion in a cohort study evaluating care in a neonatal unit after introduction of cefotaxime for five years. Given the short time it was used in large scale, the capacity of cephalosporins to induce resistance quickly was not known and was proved later.2,10,12,16
The most recent study was conducted by Yusef et al.4 This epidemiological study of the most prevalent microorganisms in a neonatal unit in Jordan compared patients infected by multidrug-resistant and sensitive bacteria. Despite the statistical significance of the association between previous use of meropenem and vancomycin with infection by multidrug-resistant microorganisms, in a multivariate analysis this association was not confirmed. However, when assessing infections with carbapenemase-producing Acinetobacter baumannii and K. pneumoniae (KPC), the exposure and time of exposure to vancomycin and meropenem presented a strong association (OR = 0.07). One of the most significant biases of this study was the use of empiric treatment of vancomycin and meropenem for late neonatal sepsis, considering their categorization as wide spectrum and second-line therapies.7,27
The CDC-recommended restriction on vancomycin use is aimed at preventing the development of vancomycin-resistant Enterococcus (VRE), which is considered a global issue because of the high mortality associated with this difficult to treat agent.12,16,36,37 Studies evaluating risk factors related to VRE infection in neonatal unit care were not found. However, there are studies associating colonization by these agents, which can be considered a risk factor for infection.2,12,15,16,30,32,33,36 During an outbreak of VRE in neonatal care units, Iosifidis et al.36 and Malik et al.37 assessed risk factors associated with colonization by this microorganism, and identified previous use of ATM, mainly 2nd line agents, such as vancomycin, meropenem and cefepime, as potential risk factors.
ConclusionsDespite the low quality of evidence, the described studies demonstrated an association between inadequate use of ATM and increase of resistant HAI in neonatal units. There is significant difficulty in performing high-quality studies because of ethical issues when conducting randomized trials in this population. In addition, there are other factors associated with the occurrence of multidrug-resistant bacterial infections, such as lack of personnel training, inadequate hand washing by the staff, and inadequate sterilization of the materials and aseptic technique procedures at the units, which may be biases in these studies. Thus, new studies are warranted in order to understand bacterial ATM resistance and propose adequate treatments to improve quality of care for newborns.
What is known about this topic:
- -
Neonates’ immune systems are immature, which contributes to the occurrence of healthcare-associated infections (HAI).
- -
Difficulties in the diagnosis of neonatal sepsis leads to a tendency to over-treat neonatal sepsis.
What this study adds:
- -
Inadequate use of ATM and the increase in neonatal sepsis are related to HAI due to bacterial ATM resistance.
- -
Additional studies should be encouraged to better understand bacterial ATM resistance and propose adequate treatments to improve the quality of care for newborns.
The authors declare no conflicts of interest.
Support – Institutional Scholarship of Federal University of Minas Gerais/Fundação de Amparo a Pesquisa de MInas Gerais (FAPEMIG).