Spinal Tuberculosis (STB) represents between 1% and 2% of total tuberculosis cases. STB management remains challenging; the first-line approach consists of medical treatment, while surgery is reserved for patients with complications. No data regarding STB treatment with bedaquiline-containing regimens are available in the literature.
Case descriptionHerein, we report the case of a 21-year-old man from Côte d'Ivoire with a multidrug resistance STB with subcutaneous abscess. After approval of the hospital off-label drug committee, we started bedaquiline 400 mg daily for two weeks, followed by 200 mg three times per week, for 22 weeks, associated with linezolid 600 mg daily, rifabutin 450 mg daily, and amikacin 750 mg daily (interrupted after eight weeks). During treatment, we performed a weekly EKG. No QT prolongation was shown, but inverted T waves appeared, requiring several cardiological consultations and cardiac MRI, but no cardiac dysfunction was found. After 24 weeks, bedaquiline was replaced with moxifloxacin 400 mg daily. The patient continued treatment for another year. We performed another computer tomography at the end of treatment, confirming the cure.
DiscussionA salvage regimen containing bedaquiline proved effective in treating multidrug-resistance tuberculosis spinal infection without causing severe adverse effects. However, further studies are needed to evaluate better bedaquiline bone penetration and the correct duration of treatment with bedaquiline in MDR spinal tuberculosis.
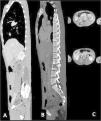
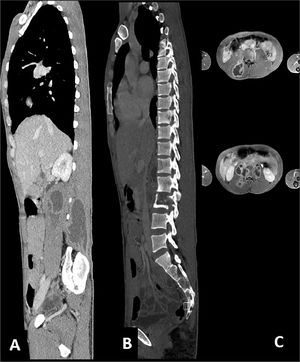
Mycobacterial musculoskeletal infections represent an uncommon extrapulmonary tuberculosis site infection. In particular, Spinal Tuberculosis (STB) represents between 1% and 2% of those cases. The disease is characterized by paradiscal destruction of a vertebra with the initial preservation of the disc. Paraspinal abscesses are often present, and they may be large at the time of presentation.
STB management remains challenging; the first-line approach consists of medical treatment, while surgery is reserved for patients with complications. Major medical challenges are represented by antibiotic resistance and drug toxicities.1-5 In addition, the limited vascular supply to parts of various bones could cause a lower drug penetration.6
Also, the duration of treatment is debated. The suggested treatment duration is 6‒9 months for regimens containing rifampicin. However, if the regimen does not contain rifampicin or in severe disease, the treatment is normally prolonged up to 12 months.7,8
Bedaquiline is an oral diarylquinoline drug with bactericidal antituberculous activity, approved to treat Multidrug-Resistant (MDR) pulmonary TB.9,10 The treatment consists of a loading dose of 400 mg daily, administered for two weeks, and a maintenance dose of 200 mg three times a week with food. It is important to know that the response to the bedaquiline depends on the minimum concentration and Area Under the Curve.11. Unfortunately, no data regarding STB treatment with bedaquiline regimens are available in the literature.
Clinical caseOur case was 21-year-old male hailing from Côte d'Ivoire. The patient presented to the emergency room for back pain after a work-related injury. The patient reported many fever episodes in the past months, so the emergency room asked us for a consultation. During the first visit, we collected physiological information and previous medical history. The patient weighed 55 kg and was 160 cm tall, with a normal body mass index (21.5 Kg/m2).
The patient told us that he arrived in Italy three years ago through the Mediterranean Route. He declared never been diagnosed with HIV, HCV, or tuberculosis before. Regarding the last few months, he reported having had many fever episodes, especially at night, and back tenderness. At the physical examination, we observed the presence of a tumefaction in the lumbar area with an external measure of 6 × 4 cm.
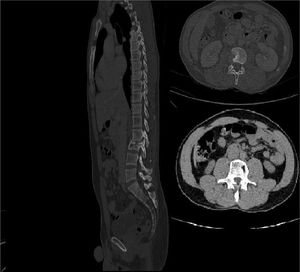
For these reasons, we decided to investigate that tumefaction with Computer Tomography (CT) scan and Nuclear Magnetic Resonance which showed liquefactive necrosis of Th10-Th11 and L2 (Fig. 1), and a large subcutaneous abscess. Several blood tests were performed, including a positive interferon-gamma release assays test, and HIV, HCV, HBV screening that turned out negative. He had an elevated C reactive protein (18 mg/dL), and a normal white blood cell count.
The abscess was drained, and a sample sent for culture. After sample decontamination, a bacterioscopic examination was positive for AFB (Acid Fast Bacillus) and Real Time-PCR (Anyplex MTB/NTM Real-Time Detection), which identified M. tuberculosis; finally, an aliquot was inoculated into MGIT and incubated until fluorescence emission in BACTEC MGIT (BACTEC MGIT 960 System ‒ Becton Dickinson). On the positive MGIT, antibiogram was performed using the AST BACTEC MGIT test to verify the sensitivity of the strain to antitubercular drugs. Then, we started treatment with Isoniazid (INH), Rifampicin (RIF), Ethambutol (EMB), and pyrazinamide (PZA). After an initial improvement, the patient reported high fever and pain. After two months, since the start of the treatment, the antibiogram showed resistance to INH, RIF, EMB, streptomycin, azithromycin, doxycycline, and susceptibility to quinolones, Linezolid (LZD), amikacin, Meropenem (MEM), and Rifabutin (RFB).
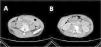
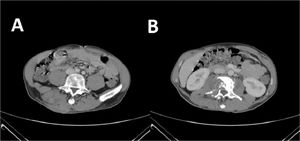
The patient was then admitted to the Infectious Diseases Unit and started treatment with MEM 2g three Times a Day (TID), amoxicillin/clavulanic acid 2.2g TID, LZD 600 mg two times a day intravenously (BID), and levofloxacin intravenously 500 mg BID. After one month of treatment, a CT scan showed a slight improvement of the abscess, but the bone injuries were unvaried (Fig. 2). We performed a second abscess sample showed a different resistance profile, now also showing resistance to meropenem. After approval by the hospital off-label drug committee, treatment with bedaquiline 400 mg daily for two weeks, followed by 200 mg three times per week, for 22 weeks, associated LZD 600 mg daily orally, RFB 450 mg daily, and amikacin 750 mg daily was started. Amikacin was discontinued after eight weeks. During treatment, a weekly Electrocardiogram (EKG) was performed. No QT prolongation was shown, but after 12 weeks of treatment, inverted T waves appeared; for this reason, several cardiological consultations and cardiac Magnetic Resonance Imaging were performed, but no cardiac dysfunction was found.
CT scan after the second treatment failure with Meropenem 2g three times a day, amoxicillin/clavulanic acid three times a day, linezolid 600 mg two times a day, and levofloxacin 500 mg two times a day. (A) presence of cutaneous abscess; (B) Presence of osteomyelitis on the second lumbar vertebra.
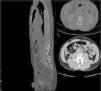
After 24 weeks, bedaquiline was interrupted and replaced with Moxifloxacin (MXF) 400 mg daily. The patients continued treatment with MXF, LZD, and RFB, for another year, without any adverse effect. At the end of treatment, we performed another CT (Fig. 3) that showed complete healing.
DiscussionNo data about the use of bedaquiline in bone tuberculosis are available in the literature. Available data on clinical trials and retrospective studies included only patients with pulmonary tuberculosis.10,12,13 Adverse events attributed to bedaquiline occurred in almost 20% of patients, and 5.8% of patients interrupting treatment. The most common adverse events were gastrointestinal symptoms, neurological symptoms, anemia, and QTc prolongation with cardiac arrhythmia.14
We performed a weekly EKG to rule out the presence of an arrhythmia. At the 12th week of bedaquiline treatment, the EKG presented inverted T waves, with no sign of myocardial ischemia. Several cardiological consultations, echocardiography, and cardiac magnetic resonance have been performed, but no dysfunctions were identified.
We decided to avoid co-administration of bedaquiline and moxifloxacin since both could cause QT prolongation, which would have lead to treatment interruption.15 However, since the patient had failed different treatments before, we decided to continue moxifloxacin, linezolid, and rifabutin until reaching 18 months of treatment.
In conclusion, a salvage regimen containing bedaquiline proved effective in treating MDR tuberculosis spinal infection without causing severe adverse effects. However, further studies are needed to better evaluate bedaquiline bone penetration and the correct duration of treatment with bedaquiline in MDR spinal tuberculosis.
FundingsThis research has not received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
CRediT authorship contribution statementAndrea De Vito: Conceptualization, Methodology, Writing – original draft, Formal analysis. Vito Fiore: Conceptualization, Methodology, Writing – original draft. Valentina Urru: Writing – review & editing, Data curation, Resources. Elena Bozzi: Writing – review & editing, Data curation, Validation. Nicholas Geremia: Writing – review & editing, Data curation, Data curation. Elija Princic: Writing – review & editing, Data curation. Donatella Canu: Writing – review & editing, Formal analysis, Validation. Paola Molicotti: . Riccardo Are: Writing – review & editing, Data curation, Validation. Sergio Babudieri: Writing – review & editing, Supervision, Validation. Giordano Madeddu: Conceptualization, Supervision, Validation, Project administration, Writing – review & editing.