Our goal was to determine the prevalence of Trichomonas vaginalis and its associated factors among women living with HIV attending an AIDS clinic in Manaus, Amazonas, Brazil.
MethodsCross-sectional study among women attending an AIDS clinic in Manaus between March and December 2010 for gynecological examination were invited to participate. Enrolled patients answered a face-to-face interview including demographic, behavioral and clinical data. They also underwent a gynecological evaluation and cervical scrape samples were collected for wet mount, Gram stain, culture and cytological analysis. A blood sample was obtained to determine TCD4+ lymphocytes and viral load.
ResultsA total of 341 (91.2%) women participated in the study. The prevalence of T. vaginalis was 4.1% (95% CI: 2.0–6.2%). Median age was 32 (interquartile range 27–38) years and median years of schooling was 9.0 (interquartile range 4–11). A total of 165 (53.2%) HIV women were classified as patients with AIDS. In multivariate analyses, squamous intraepithelial lesions in cytology [OR=2.46 (95% CI: 1.31–4.63, p=0.005)] and anal sex practice [OR=3.62 (95% CI: 1.08–12.19, p=0.037)] were associated with T. vaginalis.
ConclusionsThese results highlight that HIV-infected women should be screened for T. vaginalis. The control of this infection may have an impact on preventing reproductive complications among these women.
Sexually transmitted infections (STI) facilitate HIV transmission through direct and biological mechanisms. Early detection and treatment of STI can be an addition to HIV prevention strategies.1,2
Trichomonas vaginalis (TV) is a flagellate protozoan considered being sexually transmissible, related to low socioeconomic conditions.3 TV causes a serious discomfort to women. It is associated with adverse pregnancy outcomes, manifested by preterm rupture of membranes, preterm delivery, low-birth-weight infants,4,5 infertility,6 and cervical cancer.6,7 It also increases the transmission of human immunodeficiency virus (HIV).8–10
Among HIV-1 positive individuals, infection with TV is associated with higher genital HIV-1 levels.10,11 Antiretroviral therapy decreases genital shedding of HIV-1, but there is some evidence that shedding may still be increased in the presence of genital tract infections.12 Therefore, it has been shown that the successful treatment of trichomoniasis in antiretroviral naïve women reduces the shedding and genital HIV-1 levels.10,12
Our goal was to determine the prevalence of TV and describe the associated factors in HIV women attending an AIDS clinic in Manaus, Amazonas, Brazil.
This was a cross-sectional study conducted among women living with HIV. Women attending an AIDS outpatient clinic in Manaus for gynecological examination, between March and December 2010, were invited to participate in the study. Enrolled patients answered a face-to-face interview including demographic, behavioral and clinical data. Pregnant and postpartum women were excluded.
They also underwent a gynecological evaluation. Cervical scrape samples were collected for wet mount (in which “corkscrew” motility was observed), Gram stain, culture in Diamond medium and cytological analysis. A blood sample was obtained to determine CD4 count and HIV viral load.
Data were analyzed using the SPSS 17.0 for Windows. A preliminary analysis was performed using exploratory techniques on the data. Chi-Square, Fisher's exact and Student's t-tests were used. The odds ratio was used as a measure of association, estimated with a 95% confidence interval. Multivariate analysis was performed to estimate effects of independent variables, through the use of logistic regression models.
This study was submitted to and approved by the internal review board of the Amazonas Tropical Medicine Foundation (FMTAM). Written consent was given by the patients for their information to be stored in the hospital database and used for research.
A total of 341 (91.2%) of 374 women were included in the study. T. vaginalis was detected in 14 cases [4.1% (95% CI: 2.0–6.2%)].
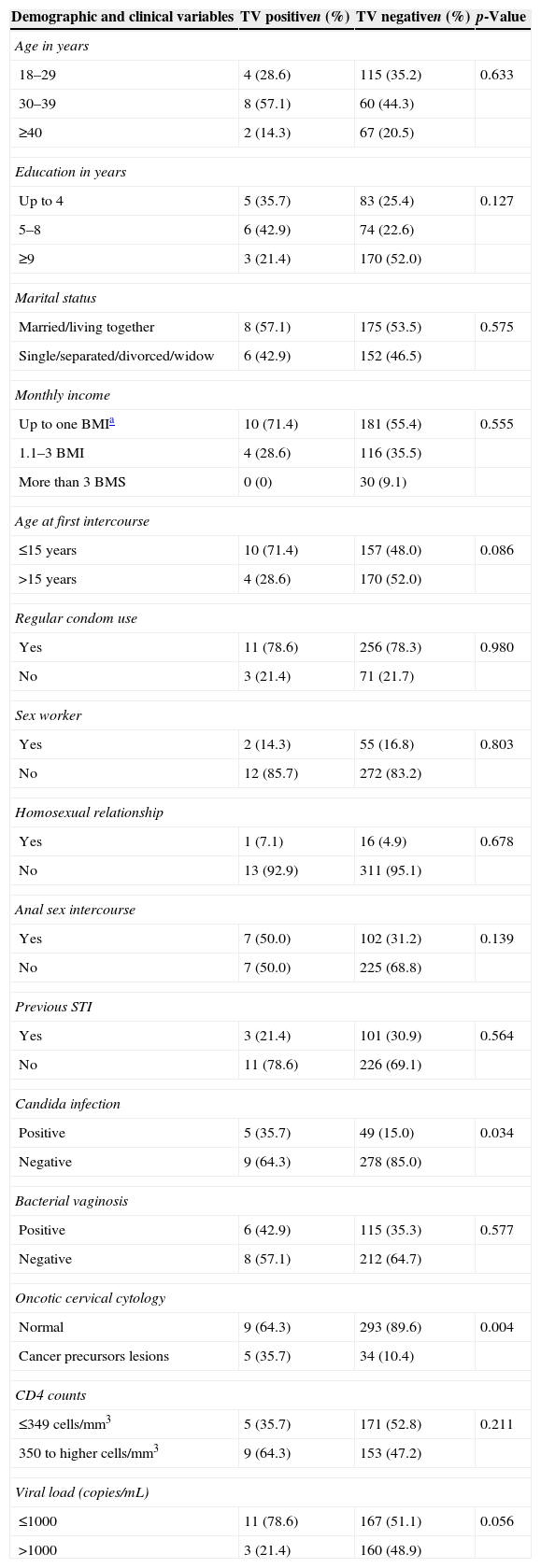
Median age was 32 (interquartile range (IQR): 27–38) years and median years of schooling was 9.0 (IQR 4–11). A total of 165 (53.2%) HIV women were classified as patients with AIDS. Table 1 shows demographic, behavioral and clinical data; 34.9% were younger than 30 years, 25.8% had up to four years of schooling and 56% had a monthly income up to US$180. Regarding sexual behavior 49% reported first intercourse up to 15 years old; 78.3% used condoms regularly in last year; 30.5% reported previous STI; 16.7% were commercial sex workers; and 32.0% reported anal sex. In univariate analysis, only candidiasis (35.7% vs. 15.0%, p=0.034) and squamous intraepithelial lesions (SIL) in cytology (35.7% vs. 10.4%, p=0.004) were significantly associated with trichomoniasis.
Demographical, behavioral and clinical characteristics of HIV women attending the AIDS clinic in Manaus, Amazonas, Brazil, (n=341).
| Demographic and clinical variables | TV positiven (%) | TV negativen (%) | p-Value |
|---|---|---|---|
| Age in years | |||
| 18–29 | 4 (28.6) | 115 (35.2) | 0.633 |
| 30–39 | 8 (57.1) | 60 (44.3) | |
| ≥40 | 2 (14.3) | 67 (20.5) | |
| Education in years | |||
| Up to 4 | 5 (35.7) | 83 (25.4) | 0.127 |
| 5–8 | 6 (42.9) | 74 (22.6) | |
| ≥9 | 3 (21.4) | 170 (52.0) | |
| Marital status | |||
| Married/living together | 8 (57.1) | 175 (53.5) | 0.575 |
| Single/separated/divorced/widow | 6 (42.9) | 152 (46.5) | |
| Monthly income | |||
| Up to one BMIa | 10 (71.4) | 181 (55.4) | 0.555 |
| 1.1–3 BMI | 4 (28.6) | 116 (35.5) | |
| More than 3 BMS | 0 (0) | 30 (9.1) | |
| Age at first intercourse | |||
| ≤15 years | 10 (71.4) | 157 (48.0) | 0.086 |
| >15 years | 4 (28.6) | 170 (52.0) | |
| Regular condom use | |||
| Yes | 11 (78.6) | 256 (78.3) | 0.980 |
| No | 3 (21.4) | 71 (21.7) | |
| Sex worker | |||
| Yes | 2 (14.3) | 55 (16.8) | 0.803 |
| No | 12 (85.7) | 272 (83.2) | |
| Homosexual relationship | |||
| Yes | 1 (7.1) | 16 (4.9) | 0.678 |
| No | 13 (92.9) | 311 (95.1) | |
| Anal sex intercourse | |||
| Yes | 7 (50.0) | 102 (31.2) | 0.139 |
| No | 7 (50.0) | 225 (68.8) | |
| Previous STI | |||
| Yes | 3 (21.4) | 101 (30.9) | 0.564 |
| No | 11 (78.6) | 226 (69.1) | |
| Candida infection | |||
| Positive | 5 (35.7) | 49 (15.0) | 0.034 |
| Negative | 9 (64.3) | 278 (85.0) | |
| Bacterial vaginosis | |||
| Positive | 6 (42.9) | 115 (35.3) | 0.577 |
| Negative | 8 (57.1) | 212 (64.7) | |
| Oncotic cervical cytology | |||
| Normal | 9 (64.3) | 293 (89.6) | 0.004 |
| Cancer precursors lesions | 5 (35.7) | 34 (10.4) | |
| CD4 counts | |||
| ≤349cells/mm3 | 5 (35.7) | 171 (52.8) | 0.211 |
| 350 to higher cells/mm3 | 9 (64.3) | 153 (47.2) | |
| Viral load (copies/mL) | |||
| ≤1000 | 11 (78.6) | 167 (51.1) | 0.056 |
| >1000 | 3 (21.4) | 160 (48.9) | |
In multivariate analyses, SIL [OR=2.46 (95% CI: 1.31–4.63, p=0.005)] and anal sex practice [OR=3.62 (95% CI: 1.08–12.19, p=0.037)] were independently associated with TV.
The prevalence of TV found in the AIDS clinic in Manaus, Amazonas was 4.1%. This result is in agreement with data described in a study conducted in an AIDS clinic in São Paulo (3.0%),13 but lower than the reported rate in other Brazilian study conducted in Recife, which investigated genital infection in HIV women and found 20% of TV.14
TV causes a wide spectrum of symptoms, ranging from a relatively asymptomatic state to severe inflammation and irritation.3,6 In this study, abnormalities at cytology and reporting anal sex were factors associated with trichomoniasis. Previous studies had described the association between TV and SIL6,7 and anal sex and higher risk of STI.15,16
Although the cross-sectional design is not the best design for determining risk factors, its use is justified for assessing the prevalence of and the associated factors for TV among HIV women. It is important to demonstrate the susceptibility of this group of women to the complications of this infection in women's health. In this study, we cannot rule out the possibility of response bias. There is always a general tendency to give socially acceptable answers.
STI continue to take an enormous toll on health, particularly on women's reproductive health. In fact, next to complications of pregnancy and childbirth, they are the leading cause of health problems for women of reproductive age.6,9 The presence of one or more STI increases the risk of becoming infected with HIV by two to nine times.1 The bidirectional relationship represents a potentially important factor in sustaining the HIV epidemic in populations where TV is endemic and it can also be affected by reproductive health tract infections.2,8,9
Health care services for HIV infected women exist and they are able to successfully control the infection and avoid the disease's progression. Although this is promising, there is still much work to be done to identify innovative interventions that refer to the social, cultural and environmental influences of the STI presence in this group. It is also necessary to find better means of access for the prevention of HIV infection, so that effective interventions can be more broadly used.
Conflicts of interestThe authors declare no conflicts of interest.