Visceral leishmaniasis (VL) or Kala-Azar (KA) is one of the most deadly forms of disease among all neglected tropical diseases. There are no satisfactory drugs or vaccine candidates available for this dreaded disease. Our previous studies showed promising therapeutic and prophylactic efficacy of the live, radio-attenuated parasites through intramuscular (I.M.) and intraperitoneal (I.P.) route in BALB/c mice model.
MethodsThe T-cell proliferation level, the mRNA expression level of inducible nitric oxide synthase (iNOS) and tumor growth factor-beta (TGF-β) genes and finally the phosphorylation levels of phosphoinositide dependent kinase 1 (PDK1), phosphoinositide 3 kinase (PI3K) and p38 mitogen activated protein kinase (p38MAPK) molecules were checked in BALB/c mice model immunized with radio-attenuated Leishmania donovani parasites through I.M. route.
ResultsHigher T-cell proliferation, increased iNOS level, and suppressed TGF-β level were found in treated infected animal groups (100 and 150Gy) in relation to untreated infected animals. Likewise, phosphorylation levels of PDK1, PI3K and p38MAPK of these two groups were increased when compared to untreated infected controls.
ConclusionThe clearance of the parasites from treated infected groups of animals may be mediated by the restoration of T-cell due to therapy with radio-attenuated L. donovani parasites. The killing of parasites was mediated by increase in nitric oxide release through PDK1, PI3K and p38MAPK signaling pathways. A lower TGF-β expression has augmented the restored Th1 ambience in the 100 and 150Gy treated animal groups proving further the efficacy of the candidate vaccine.
Visceral leishmaniasis (VL) or Kala-Azar (KA) has been ranked second to malaria in terms of mortality and fourth for morbidity.1 Global estimate is 50,000 new cases of VL each year and 90% of VL exist in Bangladesh, Brazil, India, Nepal and Sudan.2,3 The inability of KA patients to mount an anti-leishmanial cell-mediated immune response is the hallmark of the disease.4 The target pathogens are rapidly developing resistance against the available drugs including pentavalent antimonials.5 Absence of any licensed vaccine for combating the disease is another serious problem in the control management program for VL worldwide. On the other hand, the observations that people cured from VL develop protection mediated by T helper 1 (Th1) type cellular responses against new infections6 give impetus for the search of effective vaccine candidates. Invading Leishmania parasites are eliminated upon activation of macrophages by interferon-gamma (IFN-γ) secreted by Th1 cells and natural killer (NK) cells that empower them to destroy the intracellular Leishmania amastigotes by reactive nitric oxide (NO) mediated pathway.7 Inhibition of nitric oxide production from inducible nitric oxide synthase (iNOS) by the parasites makes macrophages inactive to fight against the infection.8 Any vaccine for leishmaniasis should trigger the Th1 pathway and suppress the T helper 2 (Th2) cytokines that exacerbate the disease. So far, many research groups have undertaken serious attempts in developing a successful vaccine against the disease which include use of live parasites,9 killed parasites,10 attenuated organisms,11–13 recombinant protein vaccine,14 DNA vaccines,15,16 etc. Use of live non-attenuated parasites as vaccine candidate has not been recommended by WHO for a matter of safety as reversion of virulent forms would result in adverse side effects including immunosuppression.17Leishmania parasites without virulence factor, yet maintaining the immunogenic attributes, is of great interest as attenuated parasites closely mimic the natural infection that may lead to similar immune responses without the fear of infection with live virulent parasites.18 Ionizing radiation has long been used to attenuate parasites for the purpose of vaccine development.19–24 Irradiation often causes loss or reduction of virulence and reproductive ability of the pathogens but retains their metabolic activities, morphology and antigenic profile. Thus, radio-attenuated parasites are able to trigger specific immune responses without posing risk of progressive infection.25 More interestingly, in some cases, the radio-attenuated pathogens are more immunogenic than the normal counterparts.26 Use of radio-attenuated Leishmania parasites is in practice for quite a long time now.22,27,28 Previously our group had checked the prophylactic and therapeutic efficacy of the irradiated Leishmania donovani parasites in experimental murine model and got encouraging results.29–31 The present study is an extension of earlier works to see whether there is any restoration from T cell anergy, induction of inducible iNOS and suppression of Th 2 arm of immune response by studying T cell proliferation, iNOS gene, and TGF-β gene expression, respectively. It is also an initial attempt to understand the underlying mechanism of parasite clearance by macrophages following the expressions of some signaling molecules like phosphoinositide 3 kinase (PI3K), phosphoinositide-dependent kinase 1 (PDK1) and p38 mitogen-activated protein kinase (p38MAPK), respectively, in the groups of animals receiving therapy with attenuated homologous vaccine through I.M. route.
Materials and methodsReagentsHEPES, penicillin, streptomycin, sodium bicarbonate, 2-mercaptoethanol (2-ME), bovine serum albumin (BSA), histopaque, fetal calf serum (FCS), RPMI 1640 medium, M 199 medium were purchased from Sigma–Aldrich. Anti-PI3K and anti-phospho-PI3K (p85) were obtained from Santa Cruz Biotech, Inc. All other antibodies [anti-phosphoinositide-dependent kinase 1 (PDK1) and anti-phospho-PDK1, anti-p38 mitogen-activated protein kinase (p38MAPK) and anti-phospho-P38MAPK] and LumiGlo reagents for chemiluminescence were obtained from Cell Signaling (Beverly, MA). Tritiated thymidine [3H]-thymidine (6.7Ci/mmol) was obtained from PerkinElmer®, deoxynucleoside triphosphates obtained from Promega, Taq polymerase and Moloney leukemia virus reverse transcriptase were purchased from Invitrogen and RNeasy minikit was purchased from Qiagen.
Animals, parasites and infectionBALB/c mice 4–6 weeks old with almost equal weight and same sex, reared in Institute facilities, were used for experimental purposes with prior approval of the Animal Ethics Committee of the Indian Institute of Chemical Biology, Kolkata and all animal experimentations were performed at Indian Institute of Chemical Biology, Kolkata, India following the National Regulatory Guidelines issued by Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment and Forest, Govt. of India. L. donovani isolate, AG83 (MHOM/IN/1983/AG83), originally obtained from confirmed an Indian Kala-Azar patient was used for the preparation of the live-attenuated vaccine candidate. This isolate is maintained in Golden hamsters and the promastigotes obtained after transforming the amastigotes from infected animal spleen were cultured in medium M 199 supplemented with 10% FCS along with 100U/ml penicillin and 100μg/ml streptomycin, maintained at 22°C.32
Therapy with attenuated parasitesL. donovani isolate, AG83, was attenuated by gamma irradiation from a 60Co gamma chamber with a rate of radiation of 10Gy/60s at Indian Institute of Chemical Biology, Kolkata, India. AG83 parasites (5×106/BALB/c mice) were irradiated and washed three times in sterile phosphate buffer saline (PBS) and irradiated in the gamma chamber at three different doses: 50, 100, and 150Gy respectively. The description of the animal groups for the study was as follows: Group 1, normal control animals without any infection and therapeutic immunization; Group 2, infected control animals without any immunization, while Group 3, Group 4 and Group 5 were animals that had been infected and received therapeutic immunizations with L. donovani isolate, AG83 (5×106 parasites/animal) attenuated at 50, 100 and 150Gy doses of gamma radiation, respectively, at 75 days post-infection. The treatment was repeated for three immunized groups at 15-day interval. The mice were sacrificed 15 days after the last treatment, for experimental purposes.
T-cell proliferation assayT-cell proliferation assay was performed as described previously.33 Briefly, single cell suspensions of splenocytes from different experimental groups of BALB/c mice were prepared after Ficoll density gradient centrifugation and then suspended in complete RPMI 1640. Cells were plated in triplicate at 105 cell/well concentrations in 96-well plates and allowed to proliferate for 72h at 37°C in 5% CO2 incubator in the presence of SLA concentration (5μg/ml). Eighteen hours before they were harvested in GFC membrane, cells were pulsed with 1μCi [3H]-thymidine/well. [3H]-Thymidine incorporation was measured by liquid scintillation counter (Tri-Carb 2100TR; Packard Instrument).
RNA isolation and semi-quantitative RT-PCR analyses of TGF-β and iNOS genesRNA isolation from the splenocytes was performed using the RNeasy minikit procedure (Qiagen).15 Forward and reverse primers were used to amplify the transcripts. Samples (1μg) of RNA from different experimental groups of mice were first utilized for cDNA synthesis with reverse primers (IDT; Sigma) using Mouse Moloney Leukemia Virus reverse transcriptase (Invitrogen) at 37°C for 90min. A common master mixture containing deoxynucleoside triphosphates (Promega) and Taq polymerase (Invitrogen), as well as gene specific primers and 0.25 volume of cDNA, was used for amplification with an Applied Biosystems thermocycler. The cycling conditions for genes of interest were 5min at 95°C, followed by 40 cycles of denaturation at 95°C for 30s, annealing at 56°C for 40s, and extension at 72°C for 40s. The identities of the PCR amplified gene products were verified by agarose gel electrophoresis. Identical aliquots were processed in parallel without reverse transcriptase to rule out the presence of residual genomic DNA contamination in PCR amplification preparations. Densitometric analyses were done using the Quantity One software (Bio-Rad), ethidium bromide staining, and visualization under a UV transilluminator. For densitometric calculations, the same band area was used to determine band intensity and normalized for HGPRT.
Western blot analysisSplenocytes from different groups of animals were harvested, resuspended in chilled lysis buffer (containing 20mM Tris–HCl (pH 7.5), 0.5mM EGTA, 1mM EDTA, 0.1% (vol/vol) 2-mercaptoethanol)34 and rapidly freeze-thawed thrice and passed through a 26-gauge needle (10 times) for lysis. The lysates were centrifuged (800×g for 10min at 4°C), the supernatants were collected, and the proteins were estimated using Bradford reagent. The supernatants were mixed with Laemmli buffer, heated in a boiling water bath for five min and cooled to room temperature. About 20μg of protein was loaded in lanes of Sodium Dodecyl Sulfate Poly Acrylamide Gels (SDS-PAGE) for electrophoresis. After the run, the gel was transferred to polyvinylidene difluoride membrane. Immunoblotting was performed with appropriately diluted specific primary antibodies for PI3K (anti-phosphoinositide 3 kinase Mab) and phospho PDK1 (anti-phosphoinositide-dependent Kinase 1 Mab) and p38MAPK (anti-p38 mitogen-activated protein kinase Mab) respectively. Finally, chemiluminescence was performed with LumiGlo reagent as described by the manufacturer.35 Densitometry analyses were done using Quantity1 (Bio-Rad) software.15
Statistical analysisEach experiment was performed three times and results are expressed as means±SD. Student's t-test was performed for significance and a p-value of <0.05 was considered significant. Western blots and RT-PCR show representative data from three independent experiments. * p<0.05, ** p<0.01, *** p<0.001.
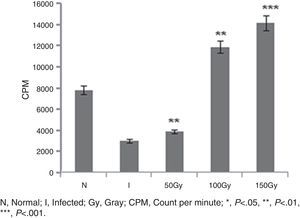
ResultsReversal of T cell responsiveness after therapy with radio attenuated parasitesCell mediated immunity is impaired in Leishmania infection characterized by marked T cell anergy to specific Leishmania antigens.15 After observing the potential therapeutic effects of 100 and 150Gy attenuated parasites in terms of reduction in organ parasite burden,31 we became interested to see whether the T cell anergy observed during progressive infection could be reversed by the treatment. Splenocytes of infected animals (Group 2) failed to mount anti-leishmanial T cell proliferation to leishmanial antigens suggesting global immune unresponsiveness at the active stage of the disease. Though Group 3 animals were treated with 50Gy radio-attenuated parasites, they also failed to do so. On the other hand, the splenocytes from treated groups (Group 4 and Group 5) stimulated with SLA showed a significantly (p<0.001) higher level (3.5–4 folds) of T cell proliferation (Fig. 1).
T cell proliferation assay in BALB/c mice groups. Number of proliferated T cells is expressed as CPM (count per minute). Proliferations of T cells were checked and compared between infected control group and treated animal groups (50, 100 and 150Gy groups), respectively. Higher T-cell proliferations compared to infected animals were found in the treated animal groups, 100 and 150Gy, respectively. Left to right, bars represent N, healthy control; I, Infected: mice received infection only; 50, 100, 150Gy represent three groups of mice treated with attenuated Leishmania donovani parasites, doses of attenuation being 50, 100 and 150Gy absorbed doses of γ-radiation, respectively. Infected (5×106 parasites/animal) and treated (twice @ 5×106 parasites per animal in 15-day interval) mice were sacrificed at 120dpi. Splenocytes were isolated and cultured in medium RPMI-1640 in 24-well plates @ 2×106 splenocytes/well. After 72h the splenocytes were pulsed with H3 thymidine and after 18h of pulsing the count was taken in liquid scintillation counter. Data represent mean±SD of six animals per group and are representative of three independent experiments; paired two-tailed Student's t-test was performed. p<0.05 was considered significant.
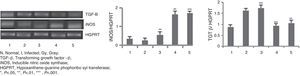
The mRNA expression levels of iNOS and TGF-β genes (Fig. 2) were checked in different animal groups by RT-PCR method. A significantly higher level of expression (six-fold, p<0.001) of iNOS mRNA was seen in animals of groups 4 and 5, whereas expression of TGF-β mRNA has been lowered by two-fold (p<0.001) in the same groups of animals compared to that of infected group of animals (Group 2). Treated animals of Group 3 did not show such increment or decrement.
RT-PCR in BALB/c mice groups for checking the expressions of iNOS and TGF-β genes. Levels of iNOS and TGF-β genes expression were compared between infected control group and treated animal groups (50, 100 and 150Gy groups), respectively. Left to right, bands and bars represent 1, healthy control; 2, Infected: mice received infection only; 3, 4, 5 represent three groups of mice treated with attenuated Leishmania donovani parasites, doses of attenuation being 50, 100 and 150Gy absorbed doses of γ-radiation, respectively. Infected (5×106 parasites/animal) and treated (twice @ 5×106 parasites per animal in 15 days interval) mice were sacrificed at 120dpi. Splenocytes were isolated from which RNA was prepared. cDNA was prepared from RNA and PCR experiments were carried out for iNOS and TGF-β gene expressions taking HGPRT gene as the housekeeping gene for internal control. Densitometry analysis was done and the ratio of iNOS and TGF-β expressed in the bar diagram. All data were compared to the infected control group. Data represent mean±SD of six animals per group and are representative of three independent experiments; paired two-tailed Student's t-test was performed. p<0.05 was considered significant. *, p<0.05; **, p<0.01; ***, p<0.001.
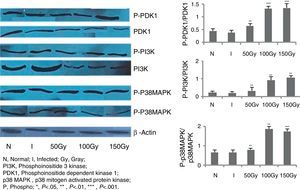
In order to score the changes, if any, in the signaling mechanisms of splenocytes of the immunized animals, immunoblot studies were carried out with PI3K, PDK1, and P38MAPK molecules (Fig. 3). These are some of the most important molecules in triggering the production of free radicals like NO and ROS. In our previous intramuscular therapeutic studies, we identified a significant increase of nitric oxide (NO) and reactive oxygen species (ROS) levels in animals of different immunized/treated groups.30,31 We measured the phosphorylation level of these three molecules in splenocytes of the animals and observed from 3.5- to 4-fold increase in phosphorylation in 100 and 150Gy animal groups compared to the infected groups (Fig. 3). This indicates the participation of these molecules in activating the effector molecules so that protection could be achieved. Animals of Group 3 that received immunization with parasites attenuated with 50Gy dose of radiation did not show any higher expression in terms of phosphorylation of any of the three signaling proteins studied here. This corroborates our previous observations where we noticed that this group of animals had no protection and succumbed to the disease.30,31
Western blot analysis to evaluate PDK1, PI3K and p38MAPK phosphorylation levels in BALB/C mice groups. Levels of PDK1, PI3K and p38MAPK phosphorylation were checked and compared between infected control group and treated animal groups (50, 100 and 150Gy groups), respectively. Splenocytes from different groups of animals were taken and the cell lysates were prepared and run in 10% polyacrylamide gels and then immunoblotted with respective monoclonal antibodies (MAbs) against non-phosphorylated and phosphorylated forms with β actin as an internal control. Phosphorylation status of each of the above mentioned molecules were expressed as the densitometric ratio of the phosphorylated form (labeled phospho or P) versus the internal control (β-actin). Left to right, bars represent N, healthy control; I, infected: mice received infection only; 50, 100, 150Gy represent three groups of mice treated with attenuated Leishmania donovani parasites, doses of attenuation being 50, 100 and 150Gy absorbed doses of γ-radiation, respectively. Infected (5×106 parasites/animal) and treated (twice @ 5×106 parasites per animal in 15 days interval) mice were sacrificed at 75dpi. Splenocytes were isolated and cultured in medium RPMI-1640 in 24-well plates @ 2×106 splenocytes/well. Representative data of three similar experiments are presented here. Data represent mean±SD value of three repeat experiments with six animals in each group; paired two-tailed Student's t-test was performed. p<0.05 was considered significant. *, p<0.05; **, p<0.01; ***, p<0.001.
We saw that attenuated parasites activated the microbicidal mechanisms of macrophages in the model host body and then the activated macrophages efficiently cleared intracellular parasites from spleen and liver cells of the protected animal groups. In our therapeutic study,31 the increased release of IFN-γ (three-fold higher than the infected group of animals) from the infected and then treated groups (100 and 150Gy) supported our hypothesis that the Th2 ambience already created in infected conditions has been overrode by the Th1 atmosphere generated by therapeutic use of radio-attenuated L. donovani. While in case of our prophylactic study,30 the radio-attenuated L. donovani parasites generated an active Th1 ambience inside the immunized host body before the host was exposed to virulent L. donovani parasites which did not allow the disease progress. The primed and then challenged groups of mice showed IFN-γ release 15-fold higher than Interleukin 4 (IL-4) and about five-fold higher than IL-10.30 Mechanistically thus both approaches are dependent either on the presence or on the establishment of Th1 milieu. Attainment of the right doses of attenuation is still a difficult task as it is directly related to the efficacy of the vaccine in one hand and to safety issues in another. If the dose of attenuation by gamma radiation is too low, there is a chance of reversion to virulent form of the pathogens causing disease progression undermining the purpose. If the dose of attenuation by gamma radiation is too high, it will immediately kill the parasites; thus, host immune response will not be evoked as live promastigotes have the potential to induce various cells to produce IFN-γ, a property which dead promastigotes lacked.36 Manna et al.37 reported that the limiting dose of attenuation by gamma radiation for L. donovani parasites is 20Krad (200Gy) up to which cells can employ homeoviscous adaptation to survive and compensate the altered conditions. In our own experiences from prophylactic and therapeutic studies, we have seen that 100 and 150Gy were the right doses of attenuation to evoke immune response in experimental hosts while 50Gy may cause reversion as evidenced from survival kinetics and parasite burden analyses of spleen and liver cells of 50Gy treated animals. Thus, extreme caution must be taken to select the right doses of attenuation.
Our previous studies had shown that BALB/c mice treated with 100 and 150Gy radio-attenuated parasites through I.M. route (and also through I.P. route) have much lower parasite burden and less hepato and splenomegaly compared to unimmunized infected animals. A significant increase in levels of free radicals, an increased Th1 cytokines profile and decreased Th2 levels in these animals compared with the infected animal groups indicated the successful clearance of intracellular parasites in these two groups, but not in 50Gy irradiated group.6 Both ROS and NO are known to be involved in parasite killing in the early stage of Leishmania infection in mice and NO alone is involved in the late phase of infection.38 ROS generation is reported to involve molecules like PI3K,39 PKC,40 ERK and Ras.38,41 P38 MAPK has been reported to be involved in the generation of NO42 while activation of P38 MAPK is dependent upon PI3K activation.43Among other associated molecules, PDK1 is an intermediate molecule in the P38MAPK activation pathway,44 and P38MAPK is well known to trigger the production of TNF-α which induces iNOS expression and NO generation.45 In our prophylactic study, we observed 3.5- to 3.9-fold higher TNF-α releases in 100 and 150Gy groups.30 In the present study, the expression of iNOS was observed to be six-fold higher in the same treatment groups. TGF-β causes the transcription of mRNA involved in immune suppression, the hall mark of full blown KA state.35 This Th2 cytokine gene expression is significantly reduced (two-fold, p<0.001) in these two groups suggesting that restoration of Th1 ambience has been initiated and established after successful therapy with radio-attenuated parasites. The 100 and 150Gy treated animals also showed a significant increase in PI3K phosphorylation, which in turn activates PDK1 phosphorylation. These are in congruence with observations by other workers.46 After activation, PDK1 could activate different PKC isotypes, which are responsible for the phosphorylation of ERK molecules that ultimately trigger ROS generation. Activated PI3K also activates P38 MAPK phosphorylation through Akt activation43 leading to NO production. In this preliminary study, we have also noticed higher level of p38 MAPK phosphorylation, which could increase TNF-α production45 and in turn induces NOS2 expression and subsequent NO generation.45
The results of the present study showed that radio-attenuated live vaccine may help the animals to recover from T cell anergy, induce NOS2 and suppress TGF-β gene expression. The NO and ROS induced parasite killing by macrophages, in groups of animals treated with radio-attenuated homologous vaccine, may follow PI3K–p38MAPK mediated NO generation in one hand and the PI3K–PDK1 mediated ROS generation on the other. Further studies are in progress.
Conflicts of interestThe authors declare no conflicts of interest.
The authors are thankful to the Council for Scientific and Industrial Research (CSIR), India for the fellowship of Sanchita Datta, CSIR Senior Research Fellow [sanction no. ACK No. 112449/2K10/1 dt 29.03.11]. The authors acknowledge the help of the Director, Public Instructions, Government of West Bengal, India and the Principal, Barasat Govt. College, Kolkata. The kind cooperation of the Director, Indian Institute of Chemical Biology, Kolkata, India is duly acknowledged.