To determine the frequency of viral pathogens causing upper respiratory tract infections in non-hospitalized, symptomatic adults in the city of Rio de Janeiro.
MethodsRespiratory samples (nasal/throat swabs) were collected between August 2010 and November 2012 and real time PCR was used to detect different viral pathogens.
ResultsViruses were detected in 32.1% (43/134) of samples from 101 patients. Specifically, 9% (12/134) were positive for HBoV, 8.2% (11/134) were positive for HAdV, 5.2% (7/134) were positive for HRV, and 1.5% (2/134) were positive for FLUBV or HMPV, as single infections. HRSV-A, HPIV-3, and HCoV-HKU1 were detected in one (0.75%) sample each. Co-infections were detected in 4.8% (6/134) of the samples. Peaks of viral infections were observed in March, April, May, August, and October. However, positive samples were detected all year round. Only 23.3% (10/43) of the positive samples were collected from patients with febrile illness.
ConclusionResults presented in this report suggest that respiratory viral infections are largely under diagnosed in immunocompetent adults. Although the majority of young adult infections are not life-threatening they may impose a significant burden, especially in developing countries since these individuals represent a large fraction of the working force.
Acute respiratory infections represent a significant morbidity and mortality burden worldwide and are caused primarily by viral infections.1–3 The high morbidity and mortality rates due to respiratory viruses have made these infections a global health concern. Since 1977, the World Health Organization has advocated the surveillance of acute viral respiratory disease programs worldwide.4 However, the majority of the data collected regarding infections in children and the epidemiology of community-acquired viral respiratory infections among adults is insufficient, perhaps with the exception of infections caused by the influenza virus. Most data available focuses on specific populations such as immunosuppressed,5–9 homeless,10,11 or elderly individuals.5,12,13
Viruses are the leading causes of acute respiratory disease throughout the world. Causative agents of respiratory disease in humans include human respiratory syncytial virus (HRSV), human parainfluenza virus (HPIV), influenza A virus (FLUAV) and influenza B virus (FLUBV), human adenovirus (HAdV), human coronavirus (HCoV), human rhinovirus (HRV), human metapneumovirus (HMPV), and human bocavirus (HBoV).1–3 In addition, two human polyomaviruses (HPyV), KIPyV and WUPyV, have been detected in patients with respiratory infections.1,2
In 2000, the Brazilian Ministry of Health established a countrywide surveillance system for respiratory viral infections. This system comprises a network of sentinel units that include outpatient clinics, emergency care departments, and general hospitals that issue a weekly report (using an online system) informing the total number of visits and the number of visits associated with influenza-like illness (ILI) (defined as a case of fever accompanied by cough or sore throat with no other diagnosis).14,15 The system is designed to detect only a small number of virus species including FLUAV, FLUBV, HRSV, HAdV, and HPIV-1, -2, and -3.14 Infections caused by other important viral pathogens such as HPMV, HRV, HBoV, and HCoV are not monitored.
The aim of this study was to determine the frequency and type of upper respiratory viral infections in non-hospitalized adults in the city of Rio de Janeiro, Brazil over a period of two years using molecular diagnostic methods.
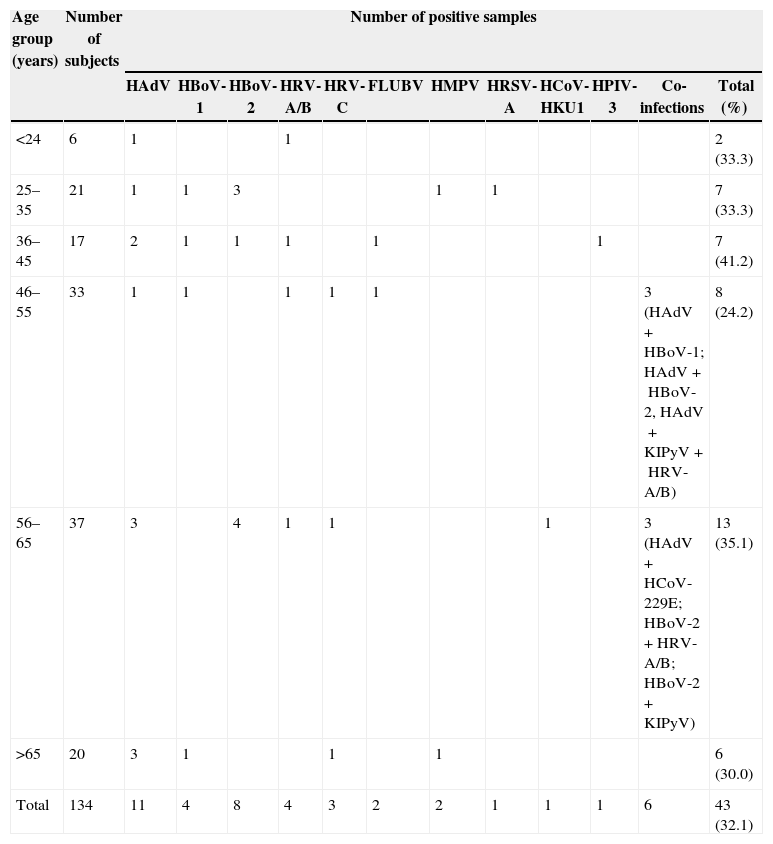
Patients and methodsImmunocompetent patients (n=134) who attended the Immunology Service of the Hospital Universitário Clementino Fraga Filho (HUCFF)/Federal University of Rio de Janeiro (UFRJ) between August 2010 and November 2012 (median age 50.5 years; ranging from 19 to 80 years) were enrolled in the present study. The age distribution of the subjects was as follows: six were 19–24 years old, 21 were 25–35 years old, 17 were 36–45 years old, 33 were 46–55 years old, 37 were 56–65 years old, and 20 were >65 years old (Table 1).
Age distribution of patients presenting with respiratory virus infections.
| Age group (years) | Number of subjects | Number of positive samples | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HAdV | HBoV-1 | HBoV-2 | HRV-A/B | HRV-C | FLUBV | HMPV | HRSV-A | HCoV-HKU1 | HPIV-3 | Co-infections | Total (%) | ||
| <24 | 6 | 1 | 1 | 2 (33.3) | |||||||||
| 25–35 | 21 | 1 | 1 | 3 | 1 | 1 | 7 (33.3) | ||||||
| 36–45 | 17 | 2 | 1 | 1 | 1 | 1 | 1 | 7 (41.2) | |||||
| 46–55 | 33 | 1 | 1 | 1 | 1 | 1 | 3 (HAdV+HBoV-1; HAdV+HBoV-2, HAdV+KIPyV+HRV-A/B) | 8 (24.2) | |||||
| 56–65 | 37 | 3 | 4 | 1 | 1 | 1 | 3 (HAdV+HCoV-229E; HBoV-2+HRV-A/B; HBoV-2+KIPyV) | 13 (35.1) | |||||
| >65 | 20 | 3 | 1 | 1 | 1 | 6 (30.0) | |||||||
| Total | 134 | 11 | 4 | 8 | 4 | 3 | 2 | 2 | 1 | 1 | 1 | 6 | 43 (32.1) |
Respiratory samples (nasal/throat swabs) were obtained from all participants. Following collection swabs were placed into viral transport media and stored at −70°C until processed. Relevant clinical information including age, sex, and clinical symptoms were collected during the medical visit using a standardized questionnaire. Respiratory illness was defined by the presence of rhinorrhea and/or cough and/or respiratory distress and/or sore throat, with or without fever.
Nucleic acid was extracted from 200μL of the collected samples using the Wizard Genomic DNA Purification KIT (Promega, Madison, WI) and the Totally RNA® Kit (Applied Biosystems/Ambion, Grand Island, NY) according to the manufacturer's instructions. Specimens were tested for the presence of FLUAV and FLUBV,16 HRSV (variant A and B),17 HPIV species 1–4,18 HRV species A and B (HRV-A/B),19 HMPV,20 HAdV,21 HBoV species 1–4,22,23 WUPyV and KIPyV,24 and HCoV species OC43, 229E, NL63, and HKU125 by real time PCR assays. Genomic RNAs of FLUV, HRSV, HPIV, HRV, HMPV and HCoV were subjected to one cycle of reverse transcription (5min at 25°C followed by 45min at 42°C) using 50pmol random primer (hexamer pd[N]6, Life Technologies, Carlsbad, CA, USA) in a Veriti 96 well (Applied Biosystems, Foster City, CA, USA) thermocycler, prior to PCR amplification. Real-time PCR amplifications were performed for each virus separately, in an ABI StepOne Real-time PCR System (Applied Biosystems). The amplification conditions consisted of 10min at 95°C, followed by 45 cycles of 10s at 95°C and 60s at 60°C. Amplification was carried out in 24μL reaction volumes, including 5μL of DNA, specific primer for each virus and 12μL of Maxima® SYBR Green qPCR Master Mix (Fermentas/Thermo Fischer Scientific, Canada). Each PCR assay for HMPV, HRV-A/B, HAdV, KIPyV or WUPyV used a single pair of primers. Multiplex assays were used to detect and discriminate the distinct species of FLUV, HRSV, HBoV, HIPV and HCoV.
A conventional RT-PCR protocol was used for detection of HRV species C (HRV-C).26 After the reverse transcription step, the viral cDNA was subjected to one step of 8min at 94°C followed by 35 cycles of PCR each consisting of 45s at 94°C, 45s at 60°C and 45s at 72°C, and a final extension step of 8min at 72°C in a Veriti 96 well thermocycler. The PCR products were analyzed by 1.2% (w/v) agarose gel electrophoresis and visualized by staining with ethidium bromide.
Positive and negative controls were included in each run. Infected cell cultures were used as positive controls for HRSV (HEp-2 cells), HPIV (Vero cells), HMPV (LLC-MK2 cells), HRV (MRC-5 cells) and HAdV (A549 cells). Positive controls of FLUAV and FLUBV consisted of allantoic fluid from FLUV infected embryonic eggs. Clinical samples of HRV-C, HCoV, KIPyV, WUPyV and HBoV, confirmed by PCR amplification and sequencing analysis, were obtained from patients with respiratory illness and used as positive controls. Negative controls consisted of viral transport medium.
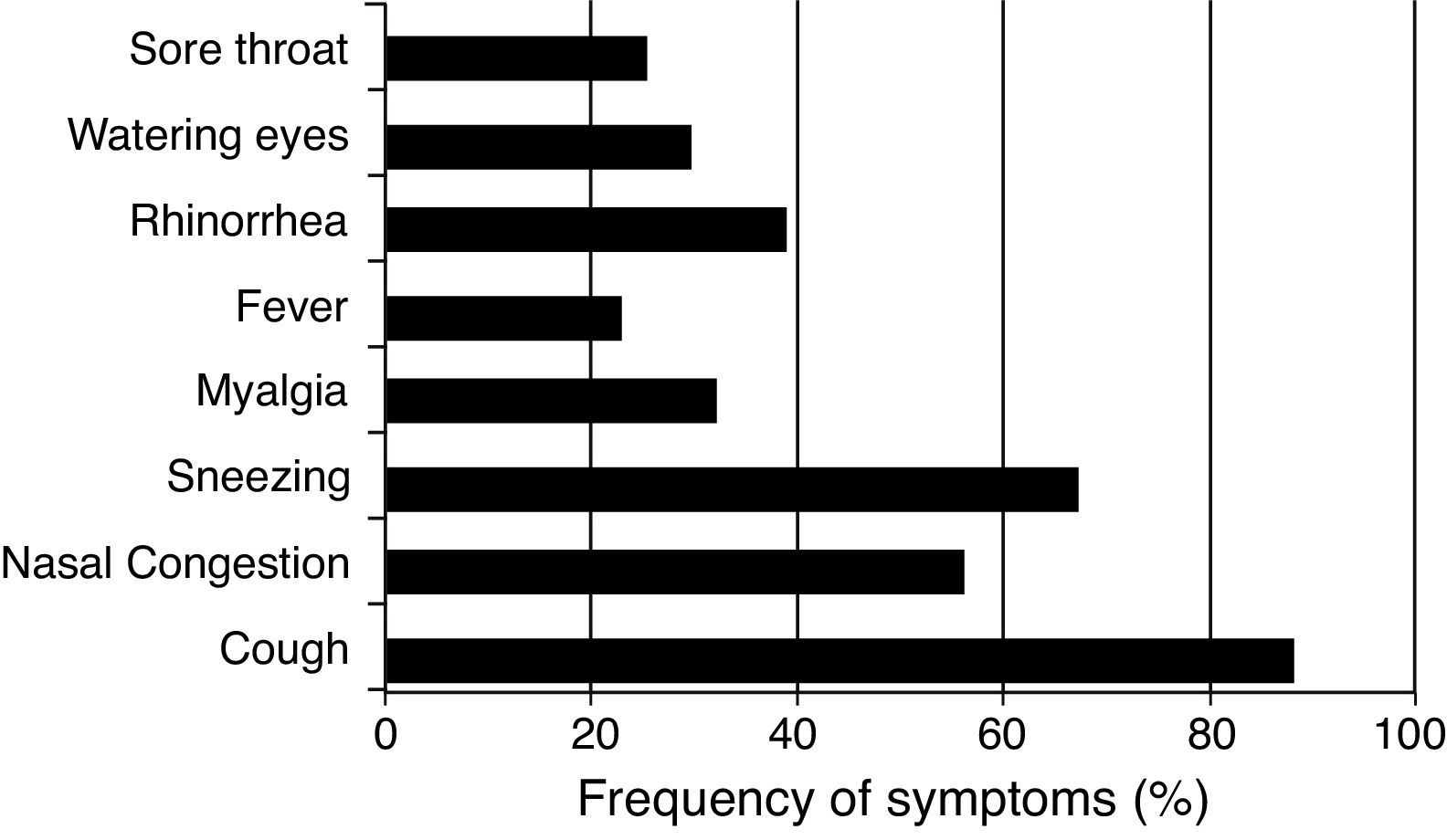
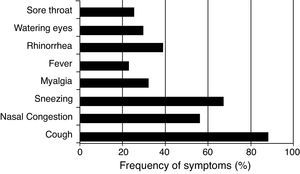
ResultsPatients (n=101) (16 male and 85 female) presenting with respiratory illness symptoms were enrolled in the present study. Respiratory samples (n=134) were collected and analyzed for the presence of viruses. The most common symptoms observed were cough (88.4%), sneezing (67.4%), and nasal congestion (58.1%). Fever was reported in 22.4% (30/134) of respiratory infection episodes (Fig. 1).
Patients were stratified into six age groups (Table 1). The overall detection frequency of any viral respiratory infections was 33.3% (2/6) in individuals <24 years old, 33.3% (7/21) among those 25–35 years old, 41.2 (7/17) among patients 36–45 years old, 24.2 (8/33) among patients 46–55 years old, 35.1 (13/37) among patients 56–65 years old, and 30.0% among patients >65 (6/20) years old. The identification rates of respiratory viruses did not differ significantly between age groups.
Co-infections occurred in six (4.5%) specimens, of which five were double infections (HAdV+HBoV1; HAdV+HBoV2; HAdV+HCoV-229E; HBoV2+HRV-A/B; HBoV2+KIPyV) and one triple infection (HAdV+HRV-A/B+KIPyV). Co-infections were only detected among patients over 45 years of age and symptoms were not different from single infected individuals.
Forty-three samples (32.1%) collected from 40 patients were positive for at least one of the viral pathogens screened for, namely 11 HAdV, 12 HBoV (four HBoV-1 and eight HBoV-2), seven HRV (4HRV-A/B and three HRV-C), two HMPV positive, two FLUVB positive, and one HRSV-A, HCoV (HKU1), and HPIV-3. Co-infections with these viruses were observed in six samples including one sample positive for KIPyV. FLUVA, HPIV-1, 2 and 4, HBoV-3 and 4, and WUPyV were not detected in any of the samples examined (Table 1). Only 23.3% (10/43) of the virus-positive samples were collected from patients with febrile illness.
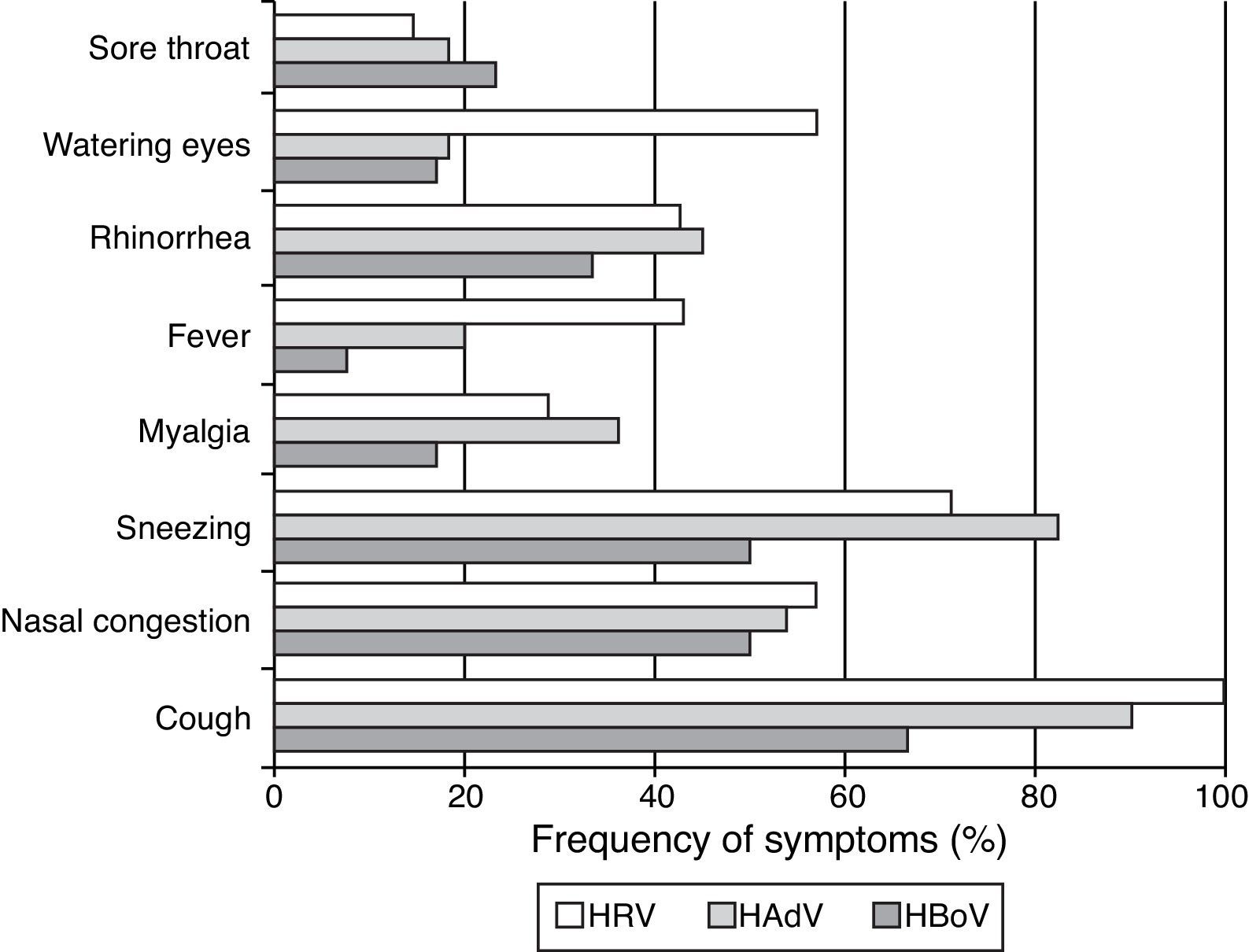
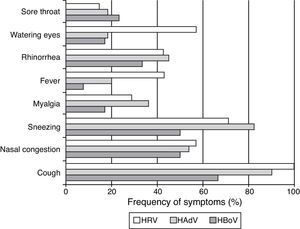
Of the seven (5.52%, n=134) patients presenting only with HRV (four positive for HRV-A/B and three for HRV-C) the most pronounced symptoms were cough (100%), sneezing (71.4%), nasal congestion (57.1%), watery eyes (57.1%), rhinorrhea (42.9%), and fever (42.9%) (Fig. 2). Symptoms were similar for infections caused by HRV-A/B or HRV-C.
HAdV was detected in samples from 11 (8.2%) patients presenting with a single infection and in samples from four patients co-infected with HBoV-1, HBoV-2, HCoV-229E, or KIPyV+HRV-A/B. The most frequent symptoms presented by patients infected with HAdV were cough (91%), sneezing (82%), nasal congestion (54.4%), and myalgia (36.4%) (Fig. 2).
Of the 16 (11.9%) patients infected with HBoV (considering both single and co-infections), five patients were infected with HBoV-1 (four single infections and one co-infection with HAdV) and 11 were infected with HBoV-2. Eight patients had single infections and three were co-infected with HRV-A/B, KIPyV, and HAdV. The most common symptoms observed among HBoV single-infected patients were cough (66.7%), sneezing (50%), nasal congestion (50%), and rhinorrhea (33.3%) (Fig. 2). Clinical symptoms were similar for HBoV-1 and HBoV-2 infections.
FLUVB was detected in two (1.5%) patients. A 45-year old female presenting with cough and myalgia and a 48-year old female presenting with cough, sneezing, watering eyes, nasal congestion, and sore throat.
Two (1.5%) HMPV infections were detected. The first case was a 69-year old female with cough, sneezing, nasal congestion, and myalgia, and the second was a 25-year old female with cough, rhinorrhea, nasal congestion, and sore throat.
HCoV-HKU1 was detected as a single infection in a 63 year-old male presenting with cough and nasal congestion. Co-infection with HCoV-229E and HAdV was detected in a 57-year old female with cough, sore throat, rhinorrhea, sneezing, nasal congestion, and myalgia.
A 31-year old male presenting with fever, cough, and sore throat was infected with HRSV-A and a 45-year old female patient infected with HPIV-3 presented with cough, rhinorrhea, sneezing, nasal congestion, and a sore throat. KIPyV was only detected in co-infections (Table 1).
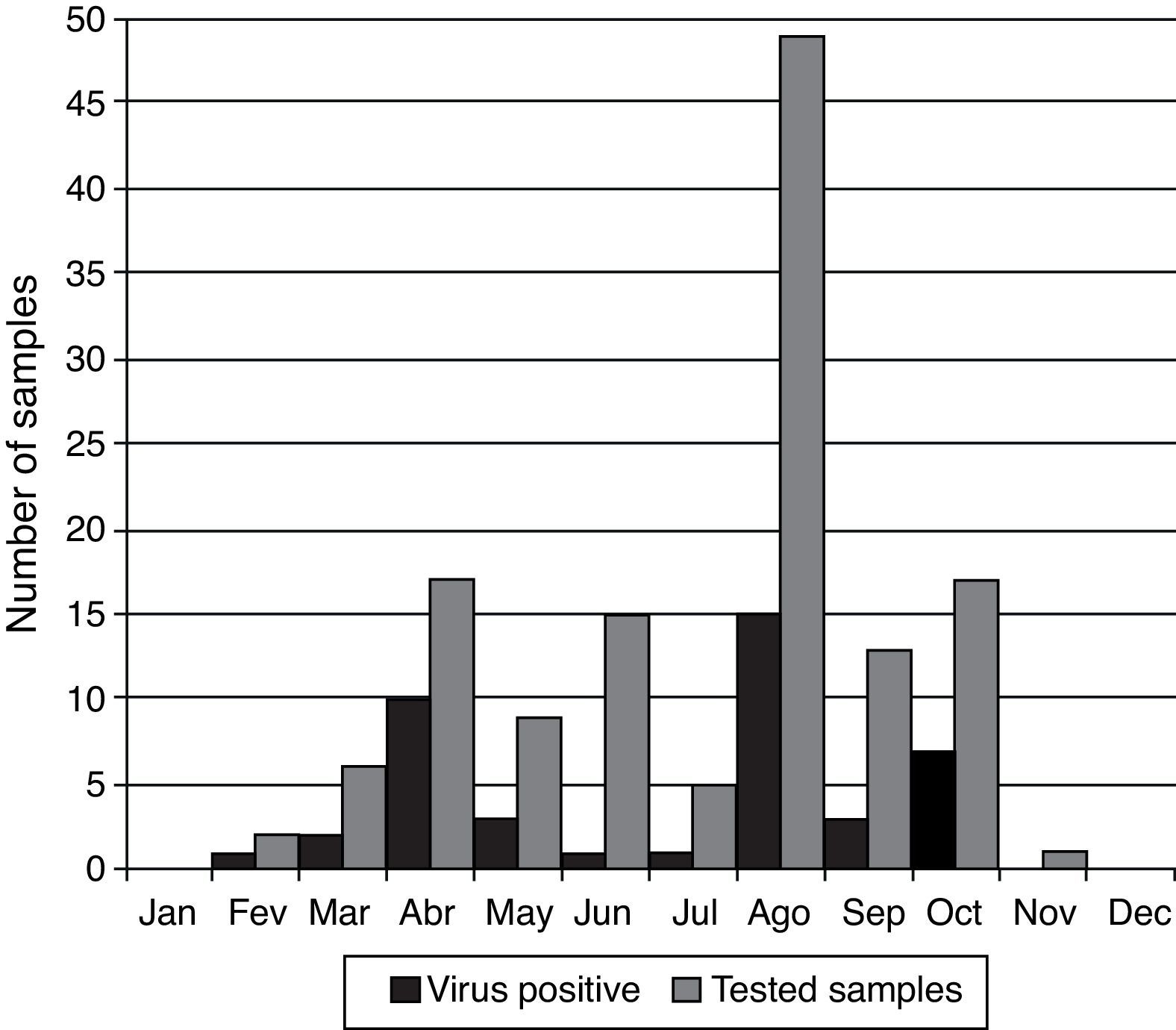
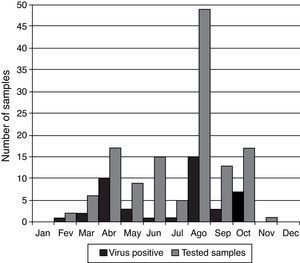
Peaks of viral infections were observed primarily during March (33.3%; n=6), April (58.8% positivity; n=17), May (33.3%; n=9), August (30.6% positivity; n=49), and October (41.2% positivity; n=17). However, viruses were detected in samples all year round. There was no sample collection during the months of January and December and only one sample was collected during November (Fig. 3).
DiscussionThe frequency of respiratory viral infections (RVIs) in immunocompetent adults is often overlooked; however, available data suggest that RVIs are responsible for a significant number of respiratory infections affecting not only the elderly (persons aged ≥65 years) but also young adults.12,13,27–32 Although the majority of infections in young adults are not life-threatening they can impose a significant burden on communities since the affected population represents a large fraction of the work force in developing countries. Data presented in this report indicate that 65.1% (28/43) of virus-positive samples were detected in patients <60 years of age.
In Brazil, most of the available data on adult RVI is restricted to immunocompromised or elderly patients with little data collected on RVIs presenting in immunocompetent adults. It has been previously demonstrated that HAdV infections were detected in 8–24.1%, HPIV-3 in 32%, HRSV in 20%, HBoV in 12%, HMPV in 12%, FLUAV in 4%, and FLUBV in 4% of transplant patients.8,33 HRV and HMPV were detected in 28.6% and 2%, respectively, in elderly patients.29 Even so, a study performed in Porto Alegre (Southern Brazil) between November 2008 and October 2010 reported that 22% of infections in immunocompetent adult patients (median age 50.6 years of age) admitted to emergence rooms with ILI or severe acute respiratory infection (SARI) were caused by respiratory viruses, mostly FLUAV and HRV-A/B. FLUVB, HAdV, HPIV, HRSV, HMPV, and HCoV were less frequently detected.34 In the present study, the overall frequency of virus detection was 32.1%. The discrepancy in detection rates could be attributed to differences in the methodologies used, for example, in the former study some samples were tested using an indirect immunofluorescence assay (IFA) and other samples were tested using conventional PCR assays compared to the use of both conventional and real time PCR assays to detect virus isolates in the present study.
The sentinel surveillance system in Brazil focuses on monitoring infections caused by FLUAV and FLUBV and other respiratory viruses such as HAdV, HPIV, HRSV, and HRV-A/B. Samples are sent to and tested in public health laboratories in each state by IFA and all positive, inconclusive, and 10% of negative samples are forwarded to one of three national reference laboratories where they are retested by PCR for respiratory viruses.14,15 Between 2000 and 2010 the Brazilian surveillance system recorded 3,291,946 cases of ILI. Of these cases, 37,120 (1% of total ILI visits) had nasopharyngeal samples collected and tested for viral infections. A total of 16,962 (45.7%) tested samples were from patients over 15 years of age with 2604 (15.4%) positive for respiratory viruses. Overall FLUAV and HPIV-3 were the most common viruses detected. However, the detection rate of FLUAV declined from 41% to 18% during this period.15 In our study, HAdV, HBoV, and HRV were the most common viruses detected. FLUBV was detected in only 1.5% (2/134) of tested samples and FLUAV was not detected.
It should be underscored that FLUV was not detected among patients over 60 years of age. The influenza vaccine in Brazil is freely distributed for at risk individuals including pregnant women of all gestational ages, women up to 45 days after childbirth, children from six months up to two years of age, people over 60 years of age, persons with chronic non-communicable diseases, native Indians, and healthcare workers.14 According to the Ministry of Health, influenza vaccine coverage in Brazil between 2010 and 2013 reached 80–88% of the target population and may explain the decline of FLUV detection over the years and the low frequency of FLUV infections found in our study. Lima et al.33 collected samples between August 2011 and August 2012 and detected FLUAV and FLUBV in only 4% of the samples using real time PCR.
Acute viral respiratory disease surveillance programs around the world monitor cases of febrile ILI, therefore patients presenting with respiratory symptoms but without fever are usually not included in surveys.15,28,35,36 In the present study we used a broader definition for respiratory illness that included patients with and without fever. Afebrile patients accounted for 76.3% of the virus-positive samples identified, suggesting that respiratory virus infections are largely undiagnosed, particularly among immunocompetent adults.
FundingThis study was supported in part by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) grant number 471063/2012-6, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and the Fundação Carlos Chagas de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) grant number E-26/103.113/2011, Brazil.
Conflicts of interestThe authors declare no conflicts of interest.
We would like to thank Soluza dos Santos Gonçalves for technical assistance.