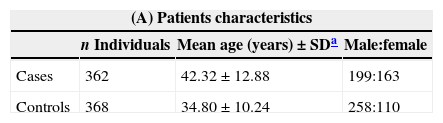Mycobacterium leprae infects skin and peripheral nerves causing deformities and disability. The M. leprae bacterium binds to ErbB2 on the Schwann cell surface causing demyelination and favoring spread of the bacilli and causing nerve injury. Polymorphisms at the ERBB2 gene were previously investigated as genetic risk factors for leprosy in two Brazilian populations but with inconsistent results. Herein we extend the analysis of ERBB2 variants to a third geographically distinct population in Brazil. Our results show that there is no association between the genotyped SNPs and the disease (p>0.05) in this population. A gene set or pathway analysis under the genomic region of ERBB2 will be necessary to clarify its regulation under M. leprae stimulus.
Leprosy is a chronic infectious disease caused by Mycobacterium leprae, and influenced by genetic and environmental factors. This infection has a broad clinical and immunological spectrum that causes high morbidity rates, with a major impact on public health. Although leprosy prevalence has been extensively reduced after the introduction of multidrug therapy and vaccination with BCG1 more than 200,000 new cases are reported annually according to World Health Organization.2 The spectrum of clinical manifestations is illustrated by two polar forms, tuberculoid and lepromatous leprosy, and various intermediate or borderline forms.3 Peripheral nerve damage, the most serious complication of leprosy, is associated with immunological and inflammatory events which evolve through time and have consequences that can extend for years after cure of the infection. The ERBB2 gene lies on chromosome 17q11-q21, and encodes an important class of receptor tyrosine kinases involved in transmission of biochemical signals.4 The binding of M. leprae to myelinated Schwann cells through ligation to the ErbB2 receptor results in Schwann cell demyelination and increases the population of de-differentiated Schwann cells through the Erk1/2 signaling pathway.5 This creates an environment that favors M. leprae proliferation and leads to nerve damage. Recent data have shown that polymorphisms in the ERBB2 gene were associated with leprosy development in families from Pará State, but not in a population from the state of Rio Grande do Norte in Brazil.6 Herein we investigate a geographically distinct population from Northeastern Brazil to further evaluate the role of this gene in leprosy susceptibility. The state of Bahia is considered of medium endemicity, according to the parameter of prevalence.7 Three hundred and sixty-two leprosy cases were selected at the Hospitals Edgard Santos and Dom Rodrigo de Menezes. Both are considered reference centers for treatment of the disease with patients diagnosed according to the guidelines of the Brazilian Ministry of Health. Additionally, 368 local blood bank donors were recruited as controls. Informed consent was obtained from all participants. Approval for the use of the samples in this study was obtained from the Federal University of Bahia (CEP-50/2010) and the Brazilian National Ethical Committee (CONEP 11019). Detailed complementary data about cases and controls are described in Table 1. Five single nucleotide polymorphisms (SNPs) (rs2517955, rs2517956, rs1810132, rs2952156, rs1136201) were genotyped by TaqMan RT-PCR technology (Applied Biosystems®) using Pre-designed genotyping assays. Table 2 describes characteristics of the SNPs used in this study and compares the minor allele frequencies between the three Northeastern populations. Tests for Hardy-Weinberg equilibrium and unconditional logistic regression analysis were done using STATA 8.2 with the freely available GenAssoc package (http://www-gene.cimr.cam.ac.uk/clayton/software/stata/) to determine allele-wise (1 df test) and genotype-wise (2 df test) associations between ERBB2 SNPs and leprosy. Our analysis did not show any significant association between the five genotyped markers and leprosy per se (p>0.05), as presented in Table 3. Nor were there any associations between ERBB2 polymorphisms and either lepromatous or tuberculoid forms of disease (data not shown).
Characteristics of case–control sample from the population of Bahia, Brazil.
| (A) Patients characteristics | |||
|---|---|---|---|
| n Individuals | Mean age (years)±SDa | Male:female | |
| Cases | 362 | 42.32±12.88 | 199:163 |
| Controls | 368 | 34.80±10.24 | 258:110 |
| (B) Clinical characteristics of the cases cohort | |
|---|---|
| Clinical phenotype | n |
| Tuberculoid (TT) | 44 |
| Borderline TT (BT) | 48 |
| Borderline (BB) | 38 |
| Borderline lepromatous (BL) | 40 |
| Lepromatous (LL) | 124 |
| Unclassified leprosy | 63 |
| Other leprosy clinical forms | 5 |
| RR | 80 |
| ENL | 84 |
| Totalb | 362 |
Note.
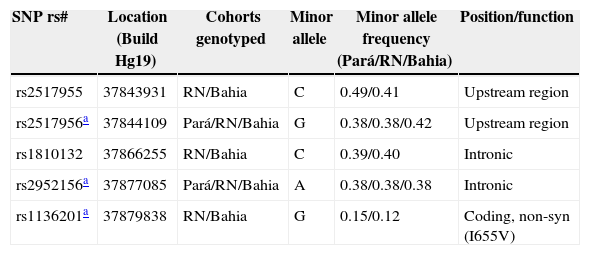
Details of ERBB2 single nucleotide polymorphisms (SNPs) genotyped in the three populations.
| SNP rs# | Location (Build Hg19) | Cohorts genotyped | Minor allele | Minor allele frequency (Pará/RN/Bahia) | Position/function |
|---|---|---|---|---|---|
| rs2517955 | 37843931 | RN/Bahia | C | 0.49/0.41 | Upstream region |
| rs2517956a | 37844109 | Pará/RN/Bahia | G | 0.38/0.38/0.42 | Upstream region |
| rs1810132 | 37866255 | RN/Bahia | C | 0.39/0.40 | Intronic |
| rs2952156a | 37877085 | Pará/RN/Bahia | A | 0.38/0.38/0.38 | Intronic |
| rs1136201a | 37879838 | RN/Bahia | G | 0.15/0.12 | Coding, non-syn (I655V) |
Note. Cohorts are from Pará, RN and Bahia states in northeastern Brazil.
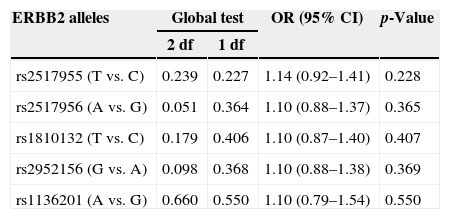
Results of logistic regression analyses for the genotyped ERBB2 polymorphisms.
| ERBB2 alleles | Global test | OR (95% CI) | p-Value | |
|---|---|---|---|---|
| 2df | 1df | |||
| rs2517955 (T vs. C) | 0.239 | 0.227 | 1.14 (0.92–1.41) | 0.228 |
| rs2517956 (A vs. G) | 0.051 | 0.364 | 1.10 (0.88–1.37) | 0.365 |
| rs1810132 (T vs. C) | 0.179 | 0.406 | 1.10 (0.87–1.40) | 0.407 |
| rs2952156 (G vs. A) | 0.098 | 0.368 | 1.10 (0.88–1.38) | 0.369 |
| rs1136201 (A vs. G) | 0.660 | 0.550 | 1.10 (0.79–1.54) | 0.550 |
Note. The 2 df test represents the genotype-wise test, and the 1 df test represents the allele-wise test. CI, confidence interval; OR, odds ratio. The OR and p-values refer to 1 df tests.
The data showed here support previous observations of a lack of association between ERBB2 genetic variants and susceptibility to leprosy per se in a similar population-based case-control data set from Rio Grande do Norte.6 Our population-based study in Bahia and that in Rio Grande do Norte had larger sample sizes than the smaller family-based study in Pará, suggesting that the association observed in the latter population may represent a false positive result. However, Araújo et al.6 draw attention to the fact that ERBB2 lies under a linkage peak8,9 for leprosy observed in the same large pedigrees employed for the study in Pará State. The region of the linkage peak at chromosome 17q11-q22 is a gene dense region of potential immunological candidate genes contributing to leprosy susceptibility.8,9 This raises the possibility that variants at ERBB2 might contribute to larger chromosome haplotypes transmitted to affected individuals in these Pará families. Further investigation is necessary to clarify if ERBB2 contributes genetically and/or functionally to leprosy susceptibility in these families. Recent data shows that M. leprae hijacks the plasticity of adult Schwann cells, to reprogram infected cells to a progenitor/stem cell-like cells as a bacterial strategy to spread infection to other tissues.10 In addition, reprogrammed cells can attract macrophages, providing evidence for a functional role of innate immune response genes during reprogramming.11 Finally, although gene analyses based on a single data type, such as gene expression data or SNP data, have successfully revealed altered cellular processes associated with different complex diseases12–15 it is also known that single variant or single gene analyses generally account for only a small proportion of the phenotypic variation in complex traits. In this sense, we have to consider that ERBB2 is located within a genetic locus that contains a number of genes directly involved in the immune response and pathogenesis of infectious diseases. A well-powered genome-wide association study would be needed to determine a role for ERBB2 relative to other genes that have been shown to achieve genome-wide significance in other populations.
Conflicts of interestThe authors declare no conflicts of interest.
Financial supportCNPq, INCT (http://www.cnpq.br).
We thank the staff of Magalhães Neto Leprosy Service, Hospital Dom Rodrigo de Menezes and HEMOBA for the logistical support in the sample collection. We also thank Dr. Selma Jeronimo for the scientific support. We thank Prof. Jenefer Blackwell for helpful comments on the manuscript.
This work was conducted with the effort and contribution of the following persons: Jamile Leão Rêgo, Joyce Moura Oliveira, Nadja de Lima Santana, Thaís Lamêgo Magalhães, Thaillamar Silva Vieira, Mayume Shibuya, Lídia Maria Machado, Paulo Roberto Lima Machado and Léa Cristina Castellucci.