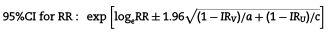Hepatitis B virus (HBV) is responsible for one of the most common human viral infections. An estimated 257 million people are living with chronic HBV infection worldwide, and mortality has reached 900,000 deaths in recent years. In 2001, the World Health Organization reported a prevalence of chronic hepatitis B infection in Iran between 2–7%.
ObjectiveTo assess the effect of the national HBV mass vaccination program after 25 years.
MethodsA retrospective cohort study was conducted in vaccinated and unvaccinated people according to the year of birth. Blood samples were obtained from each enrolled person and data about demographic variables, and medical and vaccination history were collected using a standardized questionnaire. Persons were considered uninfected if they were negative for both HBsAg and anti-HBc. Also, Vaccine effectiveness was measured by calculating the risk of disease among vaccinated and unvaccinated persons and defining the percentage risk reduction of infection in the vaccinated group.
ResultsA total of 2720 persons were interviewed. The rate of HBV breakthrough infection among the vaccinated group was significantly lower than in unvaccinated group. One hundred ninety-four cases with positive HBV markers of infection were identified. The risk ratio of HBV infection was 0.71, 95% CI: 0.54–0.94 (vaccinated/unvaccinated). The estimated vaccination effectiveness against Hepatitis B infection was 29% (95% CI: 6%–46%).
ConclusionsIran has successfully combined hepatitis B vaccination into regular immunization programs. The WHO goal of reducing HBsAg prevalence to an equivalent of 1% by 2020 has been reached. With respect to vaccination effectiveness and low prevalence of the disease in the country, catch-up hepatitis B vaccination programs for adolescents can guarantee the immunity of the population.
Hepatitis B virus (HBV) is responsible for one of the most critical human viral infections. The virus is responsible for acute and chronic liver diseases, ranging from a healthy carrier state to cirrhosis and finally hepatocellular carcinoma (HCC).1 According to the latest World Health Organization (WHO) report, 257 million people are estimated to live with chronic HBV infection worldwide, and mortality has reached to 900,000 deaths in recent years, mostly from complications such as HCC or cirrhosis.2
The overall prevalence of HBV infection in the world was estimated at 3.6%, but it depends on the geographic area. The prevalence of chronic HBV (positive HBV surface antigen or HBsAg) ranges from less than 2% in some areas with a low-prevalence situation (e.g. Western Europe) to 2–7% in intermediate-prevalence regions (eg, Mediterranean countries, and the Middle East) to more than 8% in high-prevalence areas (e.g. Western Africa).3–5
According to the reports of WHO in 2001 and of the Centers for Disease Control and Prevention (CDC) in 2005, the prevalence of chronic hepatitis B infection among Iranian population ranges between 2–7%.6,7 This wide range of prevalence is due to the different geographical areas in the country with variable customs and cultures.8–11
The important point in the management of HBV infection is that it emerges regardless of available therapeutic modalities such as interferon and antiviral therapies. There is no definite cure for HBV infection.12 On the other hand, the presence of antibody to hepatitis B surface antigen (anti-HBs) was associated with resistance to reinfection, including differing serologically-defined subtypes or serotypes.13 This raised the possibility that a neutralizing antibody either from an immune globulin or vaccine-induced might protect against HBV infection, since the hepatitis B vaccine was shown to prevent HBV infection effectively.14 Thus, strategies such as neonatal vaccination against hepatitis B, particularly those born to HBV infected mothers is the most effective strategy to diminish the spread of HBV in the community.15
In July 2016, the WHO announced its new "Global Health Sector Strategy on Viral Hepatitis, 2016-2021″ outlining a plan to reduce transmission of all hepatitis viruses and their disease burden.16 The goals for 2020 are 30% declining in new HBV infections, and also an HBsAg prevalence in children of no more than 1.0% and, by 2030, a 90% decline in new infections with a prevalence in children of no more than 0.1%. The specific goal for the prevention of mother-to-child transmission is to improve delivery of a birth dose of vaccine from the 2015 worldwide baseline of 39% to 50% by 2020 and 90% by 2030 with HBV testing in all pregnant women and development of new interventions based on antiviral treatment.16
The epidemiology of HBV has been extensively studied in the pre-vaccine era, but after introducing the vaccine, few studies have addressed this issue. On the other hand, these studies have had different results and reported the effectiveness of hepatitis B vaccination from low to high.17
In the Islamic Republic of Iran, mass vaccination against HBV infection started in 1993. This program has launched a new era of hepatitis B control and was one of the first large-scale HBV programs in the region. It was mandatory for neonates born after this time to be vaccinated using a recombinant HBV surface antigen with classic three consecutive doses. In this survey, we assessed the impact of the national HBV mass vaccination program on the chronic carriage of HBV and the rate of HBV acquisition in the Iranian population 25 years after implementation of the national vaccination program.
Materials and methodsStudy Design: retrospective or historical cohort study for the evaluation of HBV vaccination impact.
Nationwide hepatitis B vaccination programThe Ministry of Health and Medical Education in Iran held the specific program of immunization against hepatitis B for those born after 1993 through the national health system network for urban and rural areas with coverage of more than 95%.18 Infants were given intramuscular doses of HBV vaccine at birth, two and six months of age. The government has covered all the expenses of the program. In Iran, since the introduction of the national immunization program, various HBV vaccines have been used, including vaccines made in Cuba, Korea, India, and Iran.
Study population and settingIn the Islamic Republic of Iran, which has a population of 80 million, public health services are delivered through a nation-wide network. The public sector offers primary, secondary, and tertiary health services for the whole country population. The focus of the government on primary health care over the last three decades has made the public sector the main provider of primary health care services throughout the country. Some primary health care services such as prenatal care and vaccination programs are provided free of charge in public health care systems. Shiraz is the capital of Fars province in southern Iran with a population of 1,400,000, in the urban area and 650,000, in rural parts. In the urban area, nearly 100% of the population has been immunized with precise registries of vaccination data.19
Concerning the year of introduction of the national HBV vaccination program of all infants in Iran (1993), a retrospective cohort study was conducted in vaccinated and unvaccinated cohorts according to the birth year. The unvaccinated cohort was defined as persons born in 1992 and earlier. The vaccinated cohort was defined as persons born in 1994 and in the following years. The cohort born in 1993 was not included.
Inclusion and exclusion criteriaInclusion criterion in the vaccinated group was the age of 17–24 years, and in the unvaccinated group it was the age range of 26–50 years. Exclusion criteria were lack of consent for participating, having a non-Iranian nationality, or lack of documentation for HBV vaccination such as vaccination card or electronic document in those aged 17–24 years. Also, in the unvaccinated group, those who received a hepatitis B vaccine (for any reason) outside of the infantile national program were excluded from the study.
Study sampleThe sample size calculation was based on an estimated incidence of HBV infection in the Iranian population according to the last Center of Disease Control data.6 A total of 1129 participants were needed in each group, given 90% power and a two-sided significance level of 5%. Therefore, a sample size of 2256 was required for the study, and considering the attrition rate, we added 10% more in each group.
Immunization statusAn individualized investigation was conducted by trained staff. Basic information, including sex, birth date, risk assessment, occupation, medical and immunization history was collected through an interview. Immunization status was recorded from the immunization certificate or, if not available, by review of the immunization or health record. Full vaccination was defined as the receipt of three doses of hepatitis B vaccine within 12 months. All data was recorded individually.
Specimen collectionBlood samples (4 mL) were obtained from each enrolled person. Serum was separated in local laboratories, transported, and stored at −20 °C, at provincial laboratories by the cold chain service.
Laboratory testingSerum was tested for HBsAg by ELISA method (HBsAg, Dia.Pro Diagnostic Bioprobes Srl, Italy), and the antibody to hepatitis B core antigen (anti-HBc) was measured by Competitive Enzyme ImmunoAssay (HBcAb, Dia.Pro Diagnostic Bioprobes Srl, Italy).
Definition of eventsPersons were regarded as uninfected if they were negative for both HBsAg and anti-HBc. A chronic carrier was defined as a person positive for HBsAg in two different time samplings. A breakthrough infection was defined as a positive core antibody in a vaccinated person.
Vaccine effectiveness (VE) was measured by calculating the risk of disease among vaccinated and unvaccinated persons and defining the percentage reduction in risk of disease among vaccinated people in relation to the unvaccinated group.20
Data analysisAll data were double entered and analyzed with IBM SPSS Statistics for Windows version 20.0 (IBM Corp. 2011. Armonk, NY: IBM Corp.). Descriptive statistics were first provided at 95% confidence interval (95% CI). The Chi-square test was run to compare categorical variables. A p-value less than 0.05 was considered statistically significant.
According to the obtained incidence rates, the risk ratio (RR) and 95% CI were calculated, as was the vaccine effectiveness (VE) using the formula: VE = (1-RR) *100 and the 95% CI were estimated using the Taylor series,
Ethical issuesThe survey was approved by Shiraz Medical Sciences University Ethics Committee (Ethics code: IR.SUMS.REC. 1397. 437), and all study components have treated according to the national ethics regulations. Study participants were informed of study purpose, signed a written informed consent and voluntarily enrolled in the study. They were assured confidentiality.
ResultsCharacteristics of the study populationIn this study, based on the inclusion and exclusion criteria, 2720 persons (age, 17–49 years) were interviewed and provided blood samples. The mean age was 26.9 years (21.6 for vaccinated and 31.6 for unvaccinated persons), with slightly more females (56.6%), and over 50% had college education or higher (51.6%).
Table 1 describes the socio-demographic characteristics of vaccinated and unvaccinated groups. Vaccinated and unvaccinated persons did not differ in terms of a history of HBV infection of a family member (p = 0.306) and any high-risk behavior (p = 0.200).
Distribution of socio-demographic characteristics among vaccinated (N = 1273) and unvaccinated (N = 1447) cohorts.
| Frequency (%) | |||
|---|---|---|---|
| Variable | Vaccinated | Unvaccinated | p-value |
| Sex | |||
| Male | 309 (24.3) | 872 (60.3) | <0.001 |
| Female | 964 (75.7) | 575 (39.7) | |
| Education | |||
| Elementary or Middle school | 55 (4.3) | 134 (9.3) | <0.001 |
| High school | 89 (7.0) | 65 (4.5) | |
| Diploma | 558 (44.0) | 409 (28.3) | |
| Undergraduate or Bachelor | 553 (43.6) | 600 (41.6) | |
| Master of Science (MSc) | 8 (0.6) | 208 (14.4) | |
| Doctor or PhD | 6 (0.5) | 27 (1.9) | |
| History of blood transfusion | |||
| Yes | 18 (1.4) | 42 (2.9) | 0.002 |
| No | 1239 (97.7) | 1366 (95.2) | |
| I don’t remember | 11 (0.9) | 27 (1.9) | |
| History of dialysis | |||
| Yes | 0 (0.0) | 0 (0.0) | N/A* |
| No | 1267 (100.0) | 1436 (100.0) | |
| History of jaundice | |||
| Yes | 27 (2.1) | 40 (2.8) | 0.036 |
| No | 1229 (96.6) | 1360 (94.6) | |
| I don’t remember | 17 (1.3) | 37 (2.6) |
A total of 1273 (46.8%) had received full three doses of hepatitis B vaccination in the infantile period, and 1447 (53.2%) had not received hepatitis B vaccine at any age period.
For the vaccinated group, born in 1994 and afterwards, close to 100% received three consecutive doses and a timely birth dose of hepatitis B vaccine according to available health records.
Prevalence of HBsAg and Anti-HBc among people born in 1992 and earlier (unvaccinated group) and people born in 1994 and afterwards (vaccinated group)The prevalence of HBsAg among vaccinated and unvaccinated cohorts was 0.6% and 1.1%, respectively. The rates for anti-HBc were 5.5% and 7.4% among vaccinated and unvaccinated cohorts, respectively (Table 2). In our study, although the rates of HBsAg + in the two groups were not significantly different (p = 0.112), the rate of anti-HBc + was significantly lower among fully immunized people than among non-immunized persons (P = 0.041). Considering either HBsAg + or anti-HBc + or both as markers of infection,17,21,22 the rate of HBV infection among the vaccinated group (5.9%) was significantly lower than the 8.3% in the unvaccinated group (p = 0.017).
Rates of hepatitis B markers before and after starting the hepatitis B vaccination program in Iran.
| Variable | Frequency (%) | p-value | RR and 95% CI of HBV(Vaccinated/Unvaccinated) | |||
|---|---|---|---|---|---|---|
| Vaccinated | Unvaccinated | Total | ||||
| HBSAg | Positive | 7 (0.6) | 16 (1.1) | 23 (0.9) | 0.112 | 0.49 (0.20–1.20) |
| Negative | 1255 (99.4) | 1414 (98.9) | 2669 (99.1) | |||
| Anti-HBc | Positive | 69 (5.5) | 106 (7.4) | 175 (6.5) | 0.041 | 0.74 (0.55–0.98) |
| Negative | 1193 (94.5) | 1324 (92.6) | 2517 (93.5) | |||
| Hepatitis B (HBSAg + or Anti-HBc+) | Yes | 75 (5.9) | 119 (8.3) | 194 (7.2) | 0.017 | 0.71 (0.54–0.94) |
| No | 1187 (94.1) | 1311 (91.7) | 2498 (92.8) | |||
Table 3 shows the rate of HBV infection among persons with high-risk behavior history and in those reporting HBV infection among any family member. As the Table demonstrates, among unvaccinated persons there was a strong association between HBV infection and presence of hepatitis B infection in any family member (p = 0.003). However, in the vaccinated group there was no association between HBV infection and these two parameters (p = 0.738).
Factors affecting HBV infection in vaccinated and unvaccinated cohorts.
| Variable | Vaccinated | Unvaccinated | |||||
|---|---|---|---|---|---|---|---|
| Number (%) | P-Value | Number (%) | P-Value | ||||
| HBV Positive | HBV Negative | HBV Positive | HBV Negative | ||||
| History of any high-risk behaviors | Yes | 54(6.6) | 763(93.4) | 0.161 | 84(8.8) | 870(91.2) | 0.218 |
| No | 20(4.6) | 411(95.4) | 31(6.9) | 420(93.1) | |||
| History of HBV in family members | Yes | 3(6.8) | 41(93.2) | 0.738* | 9(23.1) | 30(76.9) | 0.003* |
| No | 68(5.8) | 1112(94.2) | 98(7.7) | 1178(92.3) | |||
Overall, 194 cases with markers of HBV infection were identified in the study. Of these, 119 cases occurred in unvaccinated persons and 75 cases in vaccinated persons yielding a RR of 0.71 (95% CI: 0.54–0.94). The estimated vaccine effectiveness against Hepatitis B infection was 29% (95% CI: 6%–46%) for those who received three doses at birth, two and six months with the national infantile vaccination program. On the other hand, vaccination effectiveness for the prevention of HBsAg carriage was 51%.
DiscussionHepatitis B is a serious infectious disease in Iran and our region. For that reason, we evaluated the impact of neonatal hepatitis B vaccination on HBV infection seromarkers in Iranians who were fully vaccinated at birth and compared it with an unvaccinated group. Based on the national serosurvey in 1980, prevalence of HBsAg in Iranian population was about 3.5% for all age groups, including young children.23 A review article published by Merat et al.10 in the 1980s indicated that 3% of the Iranian population were chronic HBsAg carriers. In 1996, in another national study by Zali et al., the rate of HBV carriers ranged between zero and 3.9% with an average of 1.7%.24 Older males living in rural areas with poor sanitation, low socioeconomic status, and family close contact were the main risk factors for hepatitis B infection in Iran at the time the study was conducted. Based on the results of our study, among unvaccinated individuals, HBV infection was associated with history of a family member with hepatitis B infection, but no such association was seen in the vaccinated group. One possible reason for this finding is that vaccination may not only have a direct effect on reducing HBV infection, but may also reduce the risk hepatitis B infection in family members. Therefore, it is recommended that in future studies, this relationship be investigated in more detail.
The findings from our study revealed that the mean HBsAg prevalence in the Iranian population is now close to 0.9% with a significant decline in the vaccinated population with a rate of 0.6%. In respect of the WHO goal of 1.0% prevalence of HBsAg by 2020,25,26 it seems that Iran already achieved it. Therefore, a universal infant immunization program in Iran had a major role in the control of the HBsAg carriage. These findings are compatible with other international studies.27,28 A series of long-term follow-up reports in the literature with vaccinated infants has shown that the universal immunization with the hepatitis B vaccine starting at birth has intensely reduced the subsequent development of chronic hepatitis B infection in the vaccinated population. Moreover, it affects both HBsAg carrier state and subsequent HBV complications such as HCC. These beneficial effects were detected in regions of both high endemicity27–29 and a low endemicity of HBV infection.16,25,30–33 Also, in an important report of the WHO Western Pacific Region, in 22 of 36 countries, including China, the prevalence of HBsAg positivity was 8% or more before the introduction of hepatitis B vaccination. With broadening hepatitis B vaccination coverage, including implementation of infantile vaccination program, the prevalence of HBsAg among children born in 2012 had decreased to less than 1% in 24 of 36 countries.34 Also, in another study in Malaysia, HBsAg seroprevalence among 7–12-year-old children decreased from 1.6% in 1997 to 0.3% in 2003 after implementing a universal infant vaccination program in 1990.35
In the other part of our study, we demonstrated a significant difference in the prevalence of HBV infection (by documenting HBV seromarkers) between vaccinated and unvaccinated groups. These finding confirms the results of other researchers on the effectiveness of the HBV vaccination as a means to decrease the prevalence of HBV infection and its complication such as cirrhosis and HCC.36–38 Also, our findings are consistent with those found in Italy, the pioneer of systematic vaccination in the world in 1991 in adolescents aged 12 years,39 where hepatitis B rates reduced from 5.1 per 100,000, in 1991 to 1.3 in 2005.40
In our study, the effectiveness of hepatitis B vaccination against chronic carriage of HBV was 51% 25 years after immunization, and the effectiveness against infection was 29%. This figure shows the effectiveness of the universal infancy vaccination program in Iran, but these figures are lower in comparison with other similar studies carried out in areas with high endemicity. In those areas, systematic vaccination has reduced the prevalence of HBsAg by as much as 90% in some reports.21,41 For understanding this discrepancy, we have two important explanations. First of all, there is some difference between the statistical method for the detection of vaccine effectiveness and mechanical models of vaccine effects. In other words, dynamic epidemiological models have to rely on spatiotemporally determined data, whereas epidemiological data regularly act static, and therefore are more responsive to the tools of statistics.42 So, we can find some differences between true vaccination effectiveness and calculated effectiveness in static studies. As a second explanation, advancing age has been shown to be a factor that adversely affects the immune response to hepatitis B vaccination or to other vaccines.43 Thus, after 25 years of infantile vaccination program, it is more likely for infection cases to occur which can affect vaccine effectiveness. Therefore, the present study had a lower vaccine effectiveness than studies conducted shortly after vaccination.
According to WHO recommendation, this strategy (global vaccination) should be continued and also other vaccination strategies such as vaccination in high-risk groups can be used in combination. It should be mentioned that the isolated at-risk approach is not the most effective strategy for reducing HBV prevalence, even in low-endemicity countries, as it is very labor intensive and more expensive to implement.44 It has been known that to reach a rapid impact on disease incidence, the ideal hepatitis B immunization strategy is implementation of universal vaccination in children or adolescents, or both.45
The present study had some strengths, such as cohort study design and evaluation of the long-term effects of hepatitis B vaccination among people who were sexually active.
One of the limitations of our study was resistance to be part of the study as participation was voluntary. Another probable limitation was the lack of specific data on the vaccination history in the health cards of the study participants, but this was resolved by searching for information from other available data sources, so the effect of this limitation was reduced.
ConclusionsThe present study showed the impact of the national universal infantile HBV vaccination program in Iran, and we demonstrated for the first time HBV vaccination effectiveness after 25 years of program implementation. According to our findings, Iran has successfully integrated the hepatitis B vaccine into routine immunization programs and has achieved a very significant effect on decreasing the HBsAg carrier rate among those born in 1994 and afterwards. The rate of hepatitis B surface antigen decreased gradually from 3.5% before the vaccination program to 0.6% among the vaccinated group in recent years. The WHO goal of reducing HBsAg prevalence to 1.0% by 2020 has certainly been reached. In respect to better vaccination effectiveness and minimizing the disease prevalence in the country, catch-up hepatitis B vaccination programs for adolescents before high school entrance can provide immunity for the population. At this time, our data suggest that the addition of school-based programs to universal hepatitis B immunization of infants might be helpful for further decreasing HBV infection rate in our country and its consecutive complications.List of abbreviationsHBV Hepatitis B virus World Health Organization Hepatocellular Carcinoma Hepatitis B Virus Surface Antigen Antibody to Hepatitis B Core Antigen Vaccine Effectiveness Risk Ratio
We declare that there were no conflicts of interest in this study.
Ethics approval and consent to participateThe survey was approved by the Ethics Committee of Shiraz University of Medical Sciences (Ethics code: IR.SUMS.REC. 1397. 437), and all study components have carried out according to the national ethics regulations. Study participants were informed of the purpose, signed a written informed consent (consent to participate was obtained from the participant themselves because their age range was between 17 and 49 years old) and voluntarily enrolled in the study. They were admitted to exercise confidentiality.
FundingThe study protocol was approved and financially supported by Shiraz University of Medical Sciences, Fars province, Shiraz, Iran with the code 95-7704. Shiraz University of Medical Sciences funded the process of data collection, blood sampling, preparation of kits and laboratory tests. Analysis and interpretation of data and writing the manuscript were performed by the authors.
Disclosures and acknowledgmentsWe declare that there were no conflicts of interest in this study. The study protocol has been approved and financially supported by Shiraz University of Medical Sciences, Fars province, Shiraz, Iran under the code 95-7704. The authors appreciate the collaboration of research and technology deputy of Shiraz University of Medical Sciences.
We thank the staff of the health system which provided the sera and laboratory staff at the health department in processing and testing the sera.s This study is supported by an infrastructure grant from the Shiraz University of Medical Sciences Research Deputy (Research Proposal no. 95-7704).
- Home
- All contents
- Publish your article
- About the journal
- Metrics