Studies related to infectivity status of insect vectors are seen as necessities in understanding the epidemiology of vector-borne diseases and planning effective control measures. This study assessed the infectivity ofSimulium damnosum s.l. around Owena River as well as evaluated therapeutic coverage of Ivermectin distribution in the area.
MethodHuman landing sampling method was used to collect adult flies on human attractants from 07:00 to 18:00 for two consecutive days a month for three months (July 2016 - September 2016). Parity assessment was conducted to determine the age of fly populations. Parous flies were further dissected to detect the presence or absence ofOnchocerca larvae. Biting rates and transmission potentials were calculated using standard methods. A quantitative survey was carried out to determine the therapeutic coverage and compliance to ivermectin treatment for the control of Onchocerciasis in the study area using standard household coverage questionnaires.
ResultsA total of 914 adult female flies were collected during the study period. The daily biting rate (DBR) varied from 146 fly per man day (FMD) in July to 162.5 FMD in August. The monthly biting rate (MBR) was lowest in September (2170 bites per man per month) but highest in August (3358.3 bites per man per month). MBD ranged from 13.23 fly per man hour (FMH) in July to 14.77 FMH in August. The results indicated that the majority of the flies collected at the sampling points were nulliparous [685 (74.95%)] while others were parous [229 (25.05%)]. The biting activity of the flies showed a marked decrease in population in August compared to July which later increased in September. Infection rates varied from 2 (0.7%) in July to 7 (2.2%) in August while the infectivity rate during the study ranged from zero (July and September) to 3 (1.0%) in August.
ConclusionDespite the years of treatment of onchocerciasis in Owena community, there were still some infective flies capable of transmitting O. volvolus. This could be due to the low rate of therapeutic coverage as a result of non-compliance in the community for various reasons earlier stated.
Onchocerciasis, also known as “river blindness” caused by Onchocerca volvulus is a chronic parasitic infection, a public health and socioeconomic problem of considerable magnitude in many sub-Saharan African countries.1 It is one of the five most prevalent neglected tropical diseases in the world, and is frequently treated with mass drug administration (MDA). In sub-Saharan Africa, the disease is endemic in 31 countries, although many are now working towards control and elimination.2–4
In areas of high prevalence first signs are in the skin, with chronic itching leading to secondary infection and chronic skin changes. Blindness begins slowly with increasingly impaired vision often leading to total loss of vision in young adults, in their early thirties, when they should be most productive, forcing their children to be permanently at their side to accompany them. Other effects include epilepsy and growth retardation and these are again most evident in communities with high prevalence.5
More than 99% of infected people live in 31 African countries while it also exists in some foci in Latin America and Yemen. The Global Burden of Disease Study estimated in 2017 that there were 20.9 million prevalent O. volvulus infections worldwide with 14.6 million of the infected people having skin disease and 1.15 million having vision loss. In 2017, more than 142 million people were treated in Africa where the strategy of Community Directed Treatment with Ivermectin was implemented, representing approximately 69.6% coverage of those requiring treatment globally6 and about 33 million Nigerians were at risk of onchocerciasis.7
In Nigeria, O. volvulus is transmitted primarily by the blackfly vector, Simulium damnosum complex,8,9,10 and it is highly endemic, occurring in 32 states and in Abuja, The Federal Capital Territory; it is estimated to be responsible for 200,000 cases of blindness.11 The Simulium damnosum Theobald complex are the only vectors of Onchocerca volvulus, filarial parasite causing the human onchocerciasis in West Africa.12 Onchocerciasis disease may be mild (dermatitis) or severe leading to visual impairment and blindness; it is caused by the human immune response to microfilaria released by female adult worms as they move across subcutaneous tissue and spread throughout the body. Humans are the only known reservoirs.13
Since 1995, the African Program for Onchocerciasis Control (APOC) has coordinated interventions in 16 countries where onchocerciasis is considered a public health problem through continued support of annual ivermectin treatment in affected communities as its primary focus based on community-directed strategy.14
As at 2010, therapeutic coverage has reached about 73% (75.8 million mectizan treatments) and the therapeutic coverage is expected to increase to 78% which is estimated to be at least 92.5 million treatments by year 2015.14 As a result of these sustained ivermectin treatment activities, nearly all endemic areas in Africa are under annual ivermectin treatment and the control of onchocerciasis as a public health problem has already been achieved in the majority of these areas.14 Following this success, the principal question became how long these treatments needed to continue considering that it was initially 10 years and later extended to 15–20 years; and whether in the long term it would ever be possible to eliminate onchocerciasis infection and transmission with ivermectin treatment so that treatment could be safely stopped.
Epidemiological models predicted that elimination would be feasible in the long term.14 However, in the absence of empirical evidence on the feasibility of elimination in Africa, most experts doubted that elimination would be possible in the African continent where onchocerciasis is highly endemic over vast areas, and where the vectors are highly efficient and some species can migrate over long distances.17,18 Recent epidemiological survey19 showed that after 15–17 years of ivermectin treatment in two onchocerciasis foci in Kaduna State, the prevalence had fallen to zero in all communities.
Nigeria has been administering ivermectin annually on a mass scale for over two decades and the status of transmission of the disease or impact of annual drug administration on disease transmission needs to be assessed to determine stoppage of annual MDA in line with the global road map and target for the elimination of the disease.
This study assessed the infectivity of Simulium damnosum s.l. around Owena through the evaluation of transmission indices such as the daily biting rate, monthly biting rate, proportion of parous flies, and monthly transmission potential as well as evaluated therapeutic coverage of ivermectin distribution in the area.
Materials and methodsStudy areaThe study area is located in Owena town, Ondo-East Local Government Area of Ondo State. It lies between longitude 5° 01° and 5° 45°E and latitude 7° 17° and 08° 15°N. It falls within the sub-equatorial region which is characterized by a monsoon climate. The temperature is relatively high throughout the year with an annual daily range of 20° C – 35° C, with a marked seasonal change in rainfall and relative humidity. Owena, like every other tropical area of Nigeria, has an abundant annual rainfall of over 2015mm. The population size of the community is 2842 people, made up mainly of cocoa farmers.
The Owena multipurpose dam was designed from Owena River to provide portable water to the major towns in Ondo State, including Akure, Ile-Oluji, Idanre, Ondo, Owena, Ita-Ogbolu, and Iju. In addition, the dam provides water for the irrigation of over 5,000ha of farmland. It has a storage capacity of 135-5 million cubic meters at the top water levels, 310 ordinance datum and 1670m and distributes about 60,000cm3 of water daily.
The Owena River, a fast flowing perennial river, runs closely by the Owena community and provides a conducive setting for Onchocerciasis transmission. Despite the fact that Owena multipurpose dam is very close to the community, the community has only one centrally placed tap which runs occasionally. Fishing activities by local fishermen are also currently taking place and artisans (bricklayers, bikers etc.) wash their tools within the dam lake. Residents of the area also take their bath in the lake. This therefore necessitates close contact with the river which in turn allows for greater exposure to infection. This district is considered as meso-endemic for this infection according to mapping carried out by the National rapid epidemiological mapping (REMO) conducted in 1995. However, there is paucity of published report on the prevalence of O. volvulus infection in S. damnosum in the study area despite the report20 of seasonal biting pattern of Simulium damnosum s.l. and its implications on onchocerciasis treatment with ivermectin. The study site was therefore selected because of its characteristic suitable breeding site for Simulium species and its socio-economic role.
Study designThe study was designed to evaluate entomological indices of Simulium damnosum complex using human baits at a sampling point near Owena River in Ondo.
Collection of blackfliesThe entomological evaluation of residual transmission levels was carried out in rainy seasons to determine impact of long-term treatment on the disease vector. Blood seeking female flies were collected using human landing-baits as described before21 from a sampling point near Owena River in Ondo (fly collection site). A recent study showed that flies have higher transmission rates in wet than in dry season.22 The site was sampled two days a month for three months (July 2016 - September 2016).
Fly catching was conducted between 7.00 and 18.00 (11h) by two volunteers who were treated with ivermectin before fly collection activities. The volunteers were dressed in knickers exposing the lower legs. They sat at a place close to the breeding site with the feet and the legs below the knees exposed. Female flies perching on the exposed parts were collected before it started feeding by inverting a small tube over it and replacing the cap immediately. The tubes containing the flies were labelled to indicate time and date of collection and the total number of flies caught per hour per day was recorded. The flies captured were packed in an ice box containing ice packs to stop further development of microfilariae in the flies and then transported to the laboratory for dissection.
Dissection of the blackfliesThe captured blackflies were anaesthetized with chloroform and dissected fresh to determine their physiological age (parity).9,23 The head, thorax, and abdomen of each Simulium fly was dissected separately using fine needles and forceps.24,23,20
Determination of parous rateThe ovaries of the dissected female Simulium flies were stretched to determine the elasticity of the ovaries, absence or presence of abdominal fat bodies and the thinning out and changed appearance of the malpighian tubules. Flies were recorded as nulliparous indicating that they have not undergone a complete gonotrophic cycle if the ovaries were small, compact, clear and fell apart when teased with dissecting needles. Nulliparous Simulium were also identifiable by their dense unbroken malphigian tubules and the presence of abdominal fat bodies. On the other hand, flies were identified as parous indicating that they had blood–fed and completed at least one gonotrophic cycle. Parous ovaries were relatively large and granular in appearance and could be easily stretched out and teased without breaking up. The parous flies were further dissected minutely to search for larvae of O. volvulus. Each division was dissected by teasing it apart in normal saline using dissecting needles and dissecting microscope, and searched carefully for O. volvulus larvae.20,25
Calculation of transmission indicesTransmission indices such as monthly population density (MPD) of Simulium damnosum complex in the study site, the daily biting rate (DBR), monthly biting rate (MBR), infective biting rate, the percentage of parous flies in the total dissected (proportion of parous flies), and monthly transmission potential (MTP) of Simulium damnosum complex were calculated according to previously published methods.21,26
(a) Monthly Population Density: The monthly population density also called the biting density of flies was calculated through the fly per man hour (FMH) using the formula:
MPD=Number of flies caught in a month
Number of catching days x time in hours
(b) Daily Biting rate (DBR)The Daily Biting Rate (DBR) was calculated by using the formula
DBR=Total number of flies caught in a month
Number of catching days
The Daily Parous Biting Rate was calculated by using the formula
DPBR=Total number of parous flies caught in a month
Number of catching days
It is expressed as flies per person per day.
(c) Monthly Biting Rate (MBR)
The monthly biting rate (MBR) was computed by using the formula:
MBR=Number of flies caught in a month×number of days in the month
Number of catching days
The Monthly Parous Biting Rate (MPBR) was calculated using the formula
MPBR=Number of parous flies caught×number of days in the month
Number of catching days
It is expressed as flies/person/month.
(d) Monthly Transmission Potential (MTP)
This index estimates the number of L3 (infective stage) that can be transmitted to a person exposed to the vector during a one month period. It is given by the formula:
MTP=MBR×Number of L3 stage larvae
Total number of blackflies dissected.
It is expressed as L3/person/month.
(e) Proportion of Parous flies
This represents the percentage of parous flies in the total dissected. It is given by the PPF=Number of parous flies × 100
Total number of flies dissected
It is expressed as a percentage.
Population Based Treatment Coverage Survey
A quantitative survey was carried out to determine the therapeutic coverage and compliance to ivermectin treatment for the control of Onchocerciasis in the study area. The survey was carried out using standard household coverage questionnaires.27
The study population resided in the village where ivermectin distribution has occurred using the community-directed treatment with ivermectin approach. The survey focused on all members in the village old enough to have started receiving ivermectin at the beginning of the program.
The first step on reaching a village was obtaining and reviewing the village distribution records. Village records yielded data on whether distribution took place, the level of coverage each year and the number of times individual household members took ivermectin. Records were also studied to determine whether there had been continuous annual ivermectin distribution in the community.
Data on the recent ivermectin drug distribution was obtained from the Owena health center and the therapeutic coverage of ivermectin was calculated by:
Therapeutic coverage=Total number of treated persons× 100% Total number of eligible persons.
The survey aimed to verify the reported therapeutic coverage of ivermectin in Owena carried out using household-based questionnaire. Questionnaire was administered to everyone normally resident in the household, recording key demographic information, if they received and swallowed ivermectin where relevant and the reason if they did not, historical ivermectin compliance, and information on side effects from taking the drugs.
If anyone was absent at the time of the household visit, the interviewer made one return visit later that day. If still not available and if possible, a household member answered on their behalf and this was recorded on the questionnaire.
Data analysisStatistical analyses were carried out using Excel and SPSS version 23. Descriptive statistics were employed to present simple frequencies of the dependent variables (ivermectin compliance and systematic non-compliance) and its distribution by sex, age, years lived in the village, history of taking the drug, and self-reported side effects experienced in the past. Chi-squared test was used to assess the association between various explanatory variables and the dependent variables, participation in the last treatment (ivermectin) round and systematic non-compliance.
Ethical approvalThe approval was obtained from Nigerian Institute of Medical Research and the community heads of the study sites. Informed consent was obtained from the human attractants after the study protocol was clearly explained to them. The human attractants were treated before and after the study with Ivermectin.
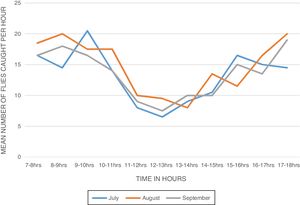
ResultsBiting activity of S. damnosum s.l. in the study siteA total of 914 female S. damnosum s.l. were caught in 6×11h (07.00–18.00) catches during the period of survey of S. damnosum s.l. in the study area. The Daily Biting Rate (DBR) varied from 146.5 flies per man per day in July to 162.5 flies per man per day in August (Table 1). The biting density of the parous flies showed bimodal peaks (Fig. 1). The first peak was observed between 8:00 - 10:00 am and the second peak occurred between 15:00 - 18:00h. The morning peak was higher than the evening peak.
Biting activity and transmission indices of S. damnosum complex in Owena.
| July | August | September | |
|---|---|---|---|
| Person days worked | 2 | 2 | 2 |
| Total number of flies caught and dissected | 291 | 325 | 298 |
| No (%) of parous flies | 99 (34.02) | 52 (16) | 78 (26.17) |
| No (%) of nulliparous flies | 192 (65.98) | 273 (84) | 220 (73.83) |
| Total No(%) of flies infected with O. volvulus | 2 (0.7) | 7 (2.2) | 3 (1.0) |
| Flies(%) with L1 and L2 of O. volvulus | 2 (0.01) | 4 (0.01) | 3 (0.01) |
| Flies(%) with L3 ofO. volvulus | 0 (0) | 3 (0.9) | 0 (0) |
| Biting density of flies | 13.23 | 14.77 | 13.55 |
| Daily Biting Rate (DBR) | 145.5 | 162.5 | 149 |
| Daily Parous Biting Rate (DPBR) | 49.5 | 26 | 39 |
| Monthly Biting Rate (MBR) | 4510.5 | 5037.5 | 4470 |
| Monthly Parous Biting Rate (MPBR) | 1534.5 | 806 | 1170 |
| Monthly Transmission Potential (MTP) | 0 | 46.5 | 0 |
| Other infections (Mermithid) | 0 | 0 | 1 |
The Monthly Biting Rate (MBR) was highest in August with 5037.5 flies per man per month while the least MBR was recorded in September with 4470 bites per man per month. The monthly biting density ranged from 13.23 in July to 14.77 in August (Table 1). During the sampling period, 229 (25.05%) of the S. damnosum s.l. caught were parous while the nulliparous female flies were 685 (74.95%) which could be due to the location of the study site. There was a marked decrease in the population of parous biting flies in August compared to July which later increased in September. The daily parous biting rate was highest in July (49.5 flies per person per day) and lowest in August (26 flies per person per day). The monthly parous biting rate ranged from 806 flies per person per month in August to 1534.5 flies per person per day in July (Table 1).
Infection rates and monthly transmission potential of S. damnosum s.l. in the study siteThe total number of infected parous flies caught with developing infective larvae as well as number of infective larvae of O. volvulus and the monthly transmission potential of O. volvulus by S. damnosum s.l. during the study period are presented in Table 1. The infection rates varied from two (0.7%) in the month of July to seven (2.2%) in the month of August while the infectivity rate during the study ranged from zero in the month of July and September to three (1.0%) in the month of August (Table 1). The monthly transmission potential of 46.5 was recorded in August (Table 1). All the infective larvae were recorded in August. Infections other than O. volvulus were recorded as ‘Other infections’ (Table 1). One of such infections was seen in September.
Population based treatment coverage surveyStudy participantsFor the quantitative survey, 425 individuals were interviewed from a total of 70 households across the village. The participants’ ages were grouped into <5 years, 5–19 years, 20–34 years, 35–49 years, 50–64 years, and 64 years and above, respectively (Table 2). The median age was 30 years (mean 31.26±19.07 years).
Demographic distribution of survey respondents, number of years lived in the village and history of ivermectin drug usage.
| Parameter | Variable | Frequency (%) |
|---|---|---|
| Age | <5 years | 35 (8.24) |
| 5–19 years | 92 (21.65) | |
| 20–34 YEARS | 120 (28.24) | |
| 35–49 years | 86 (20.24) | |
| 50–64 years | 69 (16.23) | |
| 65 years-Above | 23 (5.40) | |
| Total | 425 (100) | |
| PARAMETER | VARIABLE | FREQUENCY (%) |
| Sex | Male | 197 (46.4) |
| Female | 228 (53.6) | |
| Total | 425 (100) | |
| PARAMETER | VARIABLE | FREQUENCY (%) |
| Number of years lived in village | <5 | 84 (19.76) |
| 5-9yr | 81 (19.06) | |
| 10-14yr | 92 (21.65) | |
| 15yr-above | 168 (39.53) | |
| Total | 425 (100) | |
| PARAMETER | VARIABLE | FREQUENCY (%) |
| Have you already taken Mectizan | Yes | 246 (57.88) |
| No | 179 (42.12) | |
| Total | 425 (100) |
Of all the participants, 197 (46.4%) were males while 228 (53.6%) were females (Table 2), 341 (80.24%) had lived in the community for more than five years, 260 (61.17%) for more than 10 years, and 81 (19.06%) for less than five years. Among the participants, a total of 246 (57.88%) have taken ivermectin at least once in their lifetime while 179 (42.12%) of participants have not (Table 2).
Compliance in the last round of MDAOverall, 177 (41.65%) of participants (all ages), stated they had taken (swallowed) ivermectin during the last distribution while 248 (58.35%) of the participants had taken ivermectin during the last round. The majority of participants 105 (59.32%) that complied with ivermectin use in the last distribution were females (Table 3).
Respondents Compliance n=177 (41.65%) and non- compliance n=248 (58.35%) to Ivermectin drug usage.
| Parameter | Variable | Frequency (%) |
|---|---|---|
| Treated in the last campaign | Male | 72 (40.68) |
| Female | 105 (59.32) | |
| Total | 177 (100) | |
| Parameter | Variable | Frequency (%) |
| Not treated in the last campaign | Male | 110 (46.22) |
| Female | 128 (53.78) | |
| Total | 248 (100) |
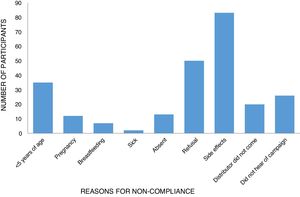
The reasons for non-compliance were related to either programmatic/delivery issues or individual factors. Nearly all participants who had not taken ivermectin during the last distribution mentioned reasons for not taking tablets at some point in the past. These included the usual exclusion criteria such as sickness (0.81%), pregnancy (4.84%), and age (14.11%). Personal reasons included being absent (5.24%), fear of side effects (33.4%), and refusal (20.5%) (Table 4). Two other key programmatic/delivery issues were the failure of the drug distributor to deliver ivermectin to the household (8.07%) and lack of awareness by the individual as to the MDA campaign (10.58%) (Table 4; Fig. 2).
Reasons for non-compliance of respondents with ivermectin drug usage.
| Parameter | Variable | Frequency (%) |
|---|---|---|
| Reason for non-compliance | <5 years of age | 35 (14.11) |
| Pregnancy | 12 (4.84) | |
| Breastfeeding | 7 (2.82) | |
| Sick | 2 (0.81) | |
| Absent | 13 (5.24) | |
| Refusal | 50 (20.16) | |
| Side effects | 83 (33.47) | |
| Distributor did not come | 20 (8.07) | |
| Did not hear of campaign | 26 (10.48) | |
| Total | 248 (100) |
The socio-demographic characteristics independently associated with drug uptake were age, sex and years of residency in the village (Tables 5 and 6). The highest compliance was found among young adults (aged 20–34 years) at 35% (Table 5). Reasons provided by the female non-compliers included fear of sterility and issues related to irregular menstruation, which they thought may be a result of taking ivermectin (Table 6).
Compliance rates with ivermectin according to age group.
| Parameter | <5 years (%) | 5-19 years(%) | 20-34 years(%) | 35-49 years(%) | 50-64 years (%) | 65 years-Above(%) | Total |
|---|---|---|---|---|---|---|---|
| Compliance | 0 (0.0) | 36 (20.3) | 62 (35.0) | 38 (21.5) | 27 (15.3) | 14 (7.9) | 177 (100) |
| Non-compliance | 35(14.1) | 56 (22.6) | 58 (23.4) | 48 (19.4) | 42 (16.9) | 9 (3.6) | 248 (100) |
| Total | 35 | 92 | 120 | 86 | 69 | 23 | 425 |
A total number of 369 of the participants covered by the study were eligible to take ivermectin during the last drug distribution campaign. According to the local Disease Control Officers in the study area, the most recent ivermectin MDA distribution was in 2015. Consequently, of the eligible participants, 177 took ivermectin in the last drug distribution campaign, which translates a therapeutic coverage of 48.0%.
Discussion
The study showed a progressive increase in fly relative abundance from July to August. This is in agreement with a previous study9 that recorded a marked increase in the fly relative abundance from July to August and a decrease in September. The study recorded the highest number of flies in August in contrast with other reports which observed the highest number of flies in September in Cross River State, Nigeria,11 Akwa Ibom State, Nigeria,25 and in Osun State, Nigeria.28
The observed decrease in fly population in Owena from August to September may be attributed to continuous flooding of the river during the peak rainy season (August and September) which may have resulted in dislodging and washing away of immature stages of Simulium flies to long distances away from Owena. These may lead to a decrease in the emergence of the flies adult population. A study showed that stormy weather may be a factor because it may have washed away most of the breeding sites, thus resulting in a smaller fly population.10 The Kwa fall in Akwa Ibom southern Nigeria was completely flooded in the month of October 1999.23 The flooding brought about a reduced productivity of existing breeding sites during the rainy season hence decreasing the fly population in the month. An other study, few flies were caught at Mayo Galke causeway in northern Cameroon when the flow of water was more than 100m3sec−1 at the height of the rainy season.29 Furthermore, the River Vina, near the Tonboro, the biting densities were at their lowest level during the rainy season in October 1976.
The diurnal biting pattern of the flies showed a bimodal peak of activity - a morning peak and an evening peak in all the months. The morning peaks occurred between 8.00am - 10.00am and the evening peaks occurred between 3:00pm - 6:00pm. These findings are in agreement with other studies conducted in Owena,9,30,31,10,25 but contradict other report in which three biting peaks in two different sampling points in south western Nigeria were observed,28 and another report that showed a unimodal biting peak activity pattern in Liberia.32 The cause of biting activity peaks is still poorly understood, but it has been suggested that an innate clock rhythm may be involved.33 Accordingly, blackflies do not suck blood daily; hence, the biting cycle may be loosely described as a circadian rhythm which by definition entails a biological rhythm on a one-day periodicity.34 Diurnal variations in the biting density have been related to variations in the temperature and humidity or to the intensity of light.35 The variation in the biting activity of the flies delimits the hours of maximum or minimum danger of transmission of onchocerciasis.29 However, several studies36,37,9,31 reported that the biting activities of S. damnosum s.l. is greatly influenced by illumination and temperature. It is possible that the bimodal peaks observed might be due to decreased illumination and temperature during these peak periods which is characteristic of climatic conditions of south-eastern Nigeria. The diurnal biting cycle of the flies may have epidemiologic implications since their biting peaks correspond to periods of peak human outdoor activities.
The relative abundance and biting patterns of the flies at Owena revealed that the control of Simulium damnosum complex with regard to their relative population density and distribution should be targeted at certain months and times of the day. For instance, larviciding will not be feasible in the months of August and September due to heavy rainfall and flooding of Owena River during these months. On the other hand larviciding and aerial spraying of insecticides in the dry season specifically in the morning and evening will go a long way in eradicating the flies and reduction in their nuisance bites and transmission of onchocerciasis.
The monthly biting density of flies recorded in Owena is far below the maximum range reported previously33 where the authors stated that in Africa the highest biting density of S. damnosum complex are usually not above 30–60 flies/month/hour in Savanna and 200 flies/month/hour in forest areas. Opara et al. recorded biting densities of 27.7 flies/month/hour, 35.8 flies/month/hour and 33.5 flies/month/hour in three different sampling points in Akwa Ibom State, Nigeria.25 The monthly biting rate in Owena can be compared with that reported by Adewale et al.9 who reported a range of 992 flies/man/month in July to 2255 flies/man/month in August, and by a range of 1860 flies/man/month in July to 2960 flies/man/month in September both around Owena Dam in Ondo State, Nigeria.20
Flies caught during the month of July had higher parous rate than those caught in the months of August and September. This contradicts the findings of a study that recorded a higher parous biting rate in September than in July.9 A high proportion of parous flies may be an indication of ageing of local blackfly populations or the presence of migratory female flies.10
The result of the dissection showed that the majority of the flies caught were nulliparous and they accounted for 74.95% of the total. This observation is consistent with other reports that recorded 53.9%, 57.86%, and 59.58% nulliparous flies in three communities along Osun River in South Western Nigeria28 and 96.83% nulliparous flies in a forest area of Cross River State, Nigeria.23 In contrast, other studies recorded 49.6%9 and 49.1%20 nulliparous flies in Owena, Ondo State, Nigeria, and 84.17% parous flies in Cross River State, Nigeria,10 86%, 89.6%, and 88.3% parous flies at three different sites in Akwa Ibom State, Nigeria25 and 74.7% parous flies in south western Ethiopia.37 The high proportion of nulliparous flies in Owena may be a reflection of local production of flies.25
The three months study recorded 12 parous flies infected with O. volvulus in the flies dissected. Nine of the flies had developing larvae of O. volvulus while three of the flies caught in August harbored the infective larvae. Hence, the infectivity rates and transmission of parasites in Owena during the months of July and September was zero while 46.5 larvae/man/month was recorded in August. This finding is in consonance with Opara et al.25 but contradicts other reports9,28 that found a monthly transmission potential of zero in the month of August. This observation confirmed that infection could be acquired during the rainy season. Coincidentally, this season is the period of active farming in most rural communities of Nigeria.25
In addition, most compounds closer to the river do not have wells where they fetch water. Hence, inhabitants go to the river to fetch water and for bathing but few who have wells do otherwise. Also, at the sampling point located close to the river, anthropogenic activities was found going on in the river and its environs throughout the study. Artisans (bricklayers, bike riders, etc.) were observed washing their tools, bikes etc. and fishermen were seen fishing.
Ivermectin has been routinely distributed in Owena since 1996. However, to date there is no evidence of interruption of transmission. There is still some uncertainty over the relative contribution of various programmatic, epidemiological and ecological factors in sustaining transmission.38 This study focused on determining the factors associated with drug coverage and compliance. The study, however, showed that the rate of compliance in the last round of ivermectin distribution was low. Poor compliance and particularly high levels of systematic non-compliance have been reported39,40 as likely contributors to the potential non-interruption of transmission in this area. In this study, 192 eligible respondents in the community were non-compliers to CDTI. This implies that members of the community who do not take the treatment may serve as a reservoir for continued transmission of onchocerciasis in the area.
The study suggests that the main reasons for individuals not taking the drugs can be broken down into two key areas, programmatic/delivery issues and individual/community factors.
Programmatic delivery issues related to low coverage included the absence of individuals on the day of the distribution, often the result of seasonal migration, a common occurrence in the study area. This is in consonance with a study conducted in Patigi, Nigeria41 which reported that absenteeism during the campaign day was the major reason for missing the treatment, as also shown in other report.38 Additionally, the CDDs failed to deliver the drug, although it was unclear whether this was a failure on the part of the CDD or was related to individual’s absence at the time of the campaign. Finally, there was unawareness of the MDA campaign by 10.48%, which could be related to poor sensitization or again linked to the migratory patterns of the population in this area. Interventions to address both issues will need to factor in future campaigns.38
Concerning individual/community level factors, fear of or experience of side effects associated with ivermectin (33.47%) was the main reason for non-compliance. Fear of side effects (often times based on previous experience of side effects) was among the reasons for non-compliance with MDA for the majority of lymphatic filariasis and onchocerciasis studies.42 Recorded side effects/adverse reactions of ivermectin from respondents included itching, rash, body pain, fever, headache, swelling, dizziness, and weakness. This is in agreement with a previous report from Ondo State, Nigeria.43 Other individual characteristics associated with non-compliance were age <5 years, pregnancy, breastfeeding, and sickness at the time of drug distribution.
Refusal to take treatment was also an important factor for non-compliance recorded in this study with some proportions (20.16%) of respondents stating taking the drug was unnecessary and general dislike of taking drugs/swallowing tablets as reasons.
The highest level of compliance recorded in those aged 20–34 years is probably related to increased enlightenment among this group. This finding contradicts a study from the West Region of Cameroon that recorded the lowest compliance in those aged 20–34 years.38 Males also showed lower compliance than females in this study, as it did in the West Region of Cameroon.38 Culturally, prescribed sex relationships influence the ways in which men and women express and experience treatment-related behaviors. Sex roles also affect participation in the program. Development of strategies that recognize these sex differences will have important implications for long-term adherence to treatment and for the overall quality and sustainability of the program.
Conclusion and recommendationDespite the years of treatment of onchocerciasis in Owena community, some infective flies capable of transmitting O. volvolus were still found. This could be due to the low rate of therapeutic coverage as a result of non-compliance in the community for various reasons stated earlier such as absence during treatment, refusals, and low awareness of the campaign. Therefore, there is need for increased enlightening campaign at the community level to enable a better therapeutic coverage rate. Further surveillance is therefore needed at the study sites and other river systems for the establishment of current status.