COVID-19 public health responses such as social distancing and community containment measures protocols are critical to preventing and containing the spread of coronavirus. Brazil accounts for almost half of Latin American HIV cases and Rio de Janeiro is the city with the second largest number of AIDS. Clinical appointments and pharmacy antiretroviral refills may be impaired due to restricted traffic and possible lockdowns, preventing people living with HIV and those using PrEP from accessing needed antiretrovirals. We hereby describe the telemedicine procedures implemented in a large PrEP delivery service in Rio de janeiro in the context of the COVID-19 pandemic. At the initial teleconsultation, individuals undergoe HIV rapid testing and are assessed by phone for PrEP related procedures. Individuals receive a digital prescription to retrieve a 120-day PrEP supply plus two HIV self-test kits. Subsequent follow-up teleconsultations will be performed remotely by phone call, including instructions for the HIV self-test performance, which results are to be sent using a digital picture. Participants will attend the service only for PrEP refill. The use of telemedicine procedures is being effective to avoid PrEP shortage and reduce the time PrEP users spend at the service during the COVID-19 pandemic and social distancing recommendations.
The HIV pandemic continues to affect a large number of individuals worldwide. In 2018, 37.9 million people were living with HIV and 1.7 million were newly infected. During the same period the Latin America region had 1.9 million accumulated cases and 100,000 new HIV infections.1 Brazil accounts for almost half of Latin American HIV cases, with a disproportional prevalence of infection among key populations, such as gay, bisexual and other men who have sex with men (MSM) (18%), and transgender women who have sex with men (TGW) (31% ).2–5 In 1996, the Brazilian government instituted universal provision of combination antiretroviral therapy (ART), with virologic and immunologic monitoring, free-of-charge to eligible HIV-infected patients.
Since December 2017 daily oral pre-exposure prophylaxis (PrEP) with emtricitabine and tenofovir disoproxil fumarate (FTC/TDF) was included in the local guidelines for populations at substantial HIV risk and provided through the Brazilian Public Health System (SUS).6 One month later, the Implementation PrEP demonstration project (ImPrEP), a cross-country study conducted in Brazil, Mexico and Peru, was initiated aiming to assess safety and feasibility of same day initiation of oral PrEP for MSM and TGW at high risk for HIV infection.7 ImPrEP is coordinated by the Instituto Nacional de Infectologia Evandro Chagas at Fundação Oswaldo Cruz (INI-Fiocruz) and 3,779 participants are under follow-up in 14 sites in Brazil, in the following locations: São Paulo (5), Rio de Janeiro (3), Brasília (1), Florianópolis (1), Manaus (1), Porto Alegre (1), Recife (1), and Salvador (1).
On March 11, 2020 the World Health Organization (WHO) first declared SARS‐CoV2 (novel coronavirus disease 2019, or “COVID-19”) a pandemic, after this disease had spread rapidly around the world since the first reports from Wuhan, China in December 2019. The burden of the pandemic tends to shift over time to poorer populations and countries due to their comparatively weak health care systems and other related issues.
COVID-19 public health responses such as social distancing and community containment measures protocols are critical to preventing and containing the spread of COVID-19, and were adopted in Brazil since March 2020 to avoid the collapse of the health system. Yet, these important measures may also affect the HIV prevention and care continuum, with reduced access to HIV testing, linkage to prevention and care services and ART or PrEP initiation and maintenance. Clinical appointments and pharmacy antiretroviral refills may also be impaired due to restricted traffic and possible lockdowns, preventing people living with HIV (PLWH) and those on PrEP from accessing needed antiretrovirals and reduce their likelihood of prevention and treatment adherence.8 Moreover, individuals may feel afraid or uncomfortable to leave their homes due to risk of COVID-19 infection.
As such, tailored strategies that minimize disruptions in access and adherence to antiretrovirals for HIV prevention and treatment are urgent.
The Brazilian Federal Council of Medicine (CFM) defines teleconsultation as a “remote medical consultation, mediated by technologies, with doctor and patient located in different geographical spaces”.9 During this pandemic, the CFM recognized the possibility and ethics of using telemedicine.10 In addition, the Brazilian Ministry of Health has extended PrEP dispensation from 90- to 120-day supplies.11 As a consequence, health services offering PrEP have been required to implement a new framework to meet the current demands.
Rio de Janeiro is the city with the second largest number of AIDS cases in Brazil. The Instituto Nacional de Infectologia Evandro Chagas INI-Fiocruz is the largest provider of HIV prevention and care services, with around 2,000 individuals using PrEP through SUS and ImPrEP. We hereby describe the telemedicine procedures implemented for PrEP delivery at INI-Fiocruz in the context of the COVID-19 pandemic.
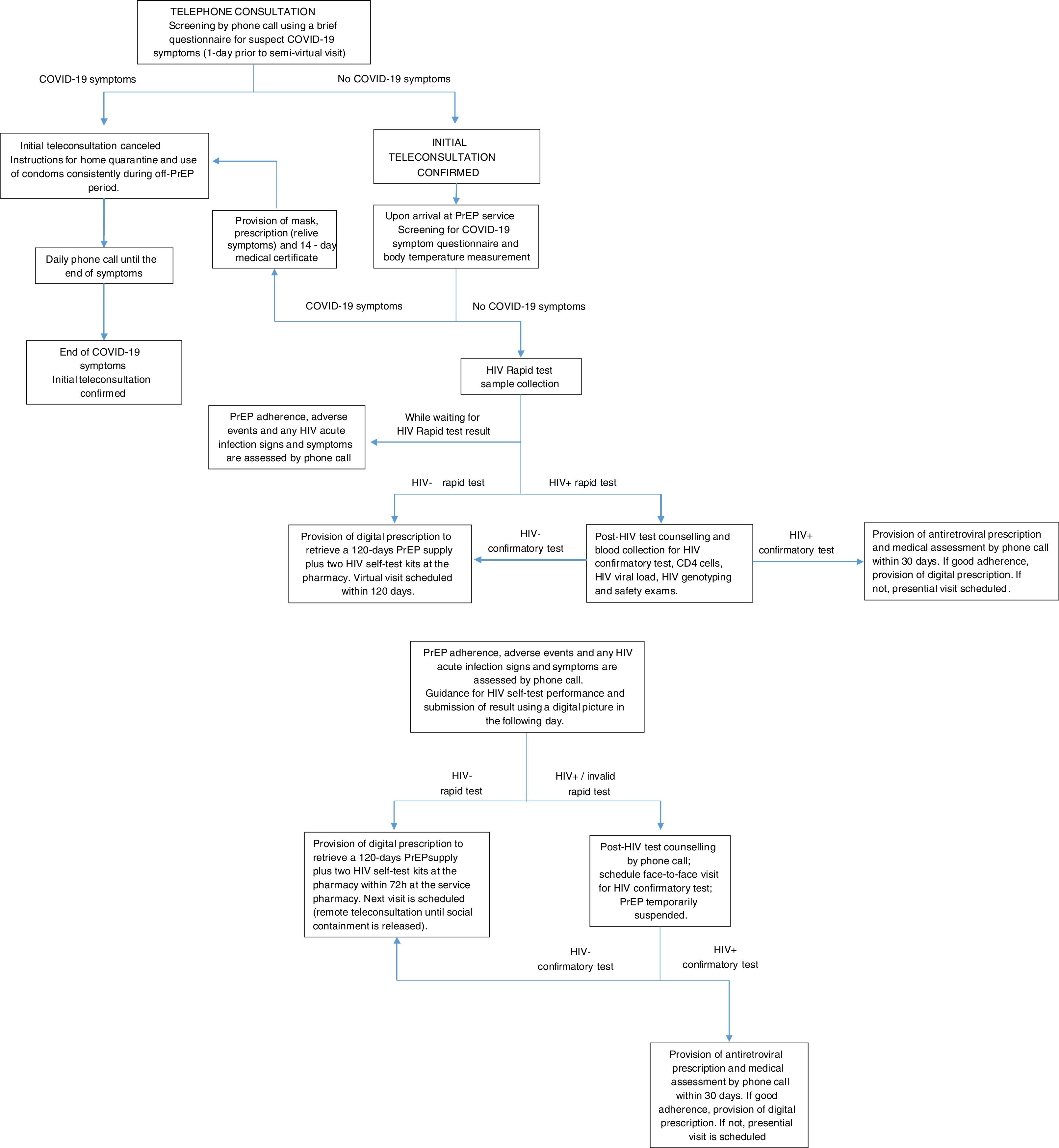
Telemedicine procedures for PrEP delivery at INI-Fiocruz were organized in three different teleconsultations: (1) telephone consultation to screen for suspect COVID-19 symptoms; (2) initial teleconsultation, in which the participant must come to the service for HIV testing and PrEP refill (Fig. 1); (3) follow-up teleconsultations, in which the participant must come to the service only for PrEP refill (Fig. 2). For all individuals enrolled in the INI-Fiocruz PrEP Program and currently using PrEP (either through SUS or enrolled in ImPrEP), an initial teleconsultation is scheduled prioritizing those whose PrEP supply will end within the following seven days. On the day prior to the initial teleconsultation, a telephone consultation is performed using a brief questionnaire to screen participants for suspect COVID-19 symptoms. Those with symptoms have their visit cancelled, receive COVID-19 targeted information and are instructed to use condoms consistently in all sexual relations during the off-PrEP period.
Upon arrival at INI-Fiocruz for the initial teleconsultation, participants are again assessed for COVID-19 symptoms using the same questionnaire. Individuals with symptoms have their visit cancelled and receive the same instructions as described for telephone consultation. Individuals without symptoms undergo HIV rapid testing and are assessed by phone for PrEP adherence, adverse events and for any HIV acute infection signs and symptoms while waiting for the HIV test result. Once the HIV rapid test result is available and is negative, they receive this information and a digital prescription to retrieve a 120-day PrEP supply plus two HIV self-test kits at the pharmacy; post-HIV test counselling is provided if requested, since everyone who is in telemedicine consultation is already on quarterly PrEP follow-up visits and is aware of the significance of the results of HIV rapid test. During the visit or between visits, the participant has the possibility to access the health professional to clarify any questions related to HIV testing or PrEP. If the HIV rapid test is positive, individuals undergo face-to-face post-HIV test counselling and blood collection for HIV confirmatory test and additional tests such as, CD4 count, HIV viral load, HIV genotyping, and safety tests. If HIV positive is confirmed, the participant receives an antiretroviral prescription in a presential medical visit and new medical assessment by phone call within 30 days.
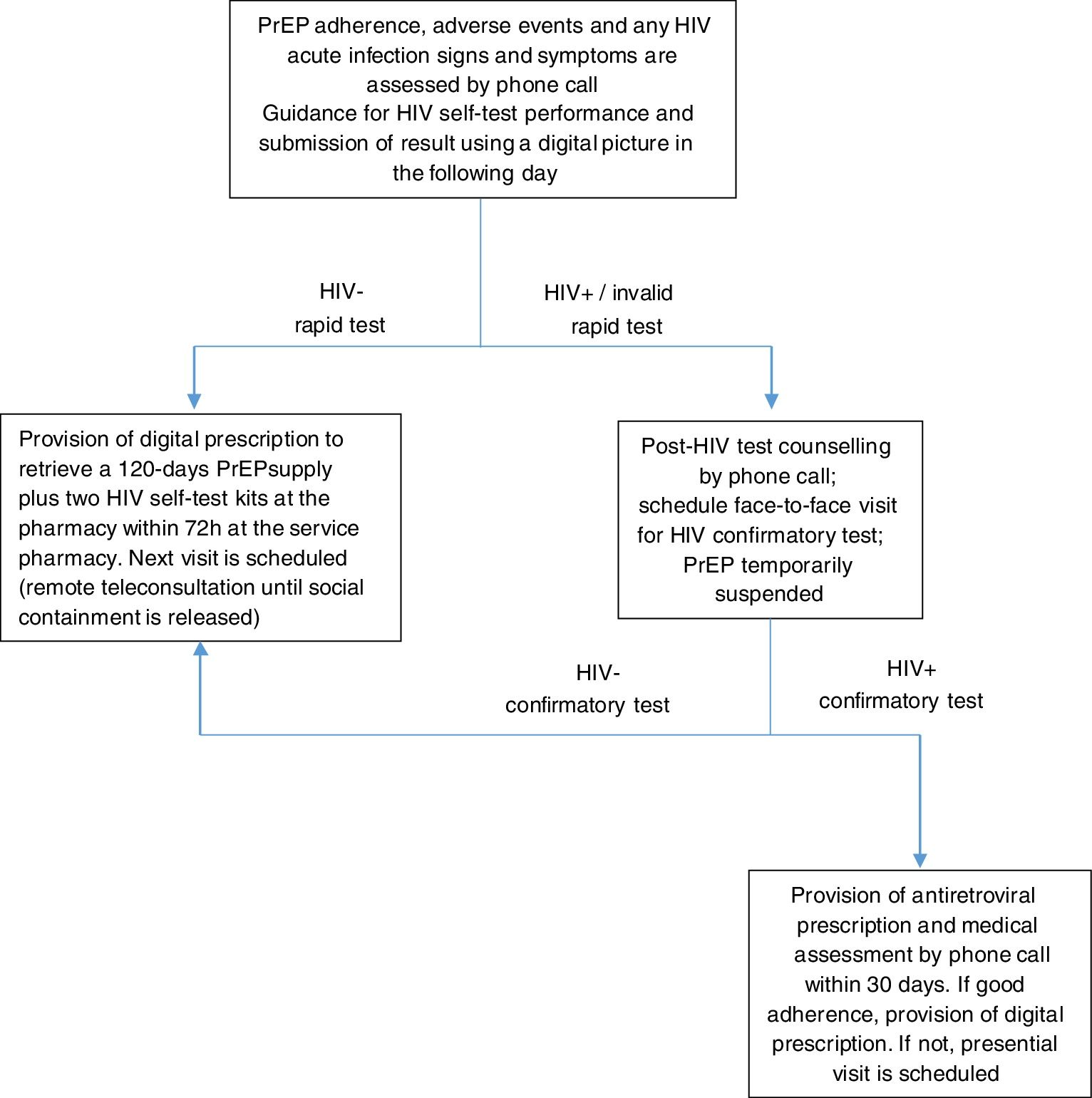
After this initial teleconsultation, until the social containment is released, all subsequent follow-up teleconsultations will be performed remotely by phone call, including instructions for the HIV self-test performance and for sending the results using a digital picture. Participants will attend the service only to collect their PrEP refill, significantly reducing the time spent at the service and the social contact. Unfortunatelly, we were unable to implement home delivery of the PrEP supply, which would have been ideal during the social distancing period, but unnafordable. PrEP users are also instructed to make phone contact in-between visits in case of symptoms of sexually transmitted infections (STI) or other health problems related to PrEP medication.
Since the implementation of the telemedicine procedures in March 23, 2020 until June 05, 2020, 564 participants completed the telephone consultation and the initial teleconsultation. The average time spent at the service was reduced in two hours (from three hours to one hour). We are in the process of collecting structured acceptability data from all individuals who had the initial teleconsultation. So far, most of the participants are reporting to be very satisfied with the new procedures.
Despite our preliminary optimistic results, the implementation of telemedicine at other PrEP services in Brazil or other middle-income countries may face constrains. First, although cell phones are widely available,12 Brazil and other low- and middle-income countries face huge social disparities, that will become even more profound during the COVID-19 pandemic and could preclude cell phone and internet availability. Second, HIV self-test availability and acceptability should be considered. In a web-based survey conducted in 2018 with more than 11,000 Brazilian MSM, 44% were willing to use HIV self-test but 87% of them considered the post-HIV test counselling support important.13–15 Thus, we reinforce the need of trained staff support during the virtual visit. Lastly, lack of regulations on telemedicine and HIV self-testing could forbid the implementation of these procedures in other low- and middle-income countries.
In conclusion, the use of telemedicine procedures in a large PrEP service is being effective to avoid PrEP shortage and reduce the time PrEP users spend at the service during the COVID-19 pandemic and social distancing recommendations. Preliminary data showing high acceptability is an indicator that telemedicine procedures could be maintained after COVID-19 pandemic and should be considered by other PrEP services.
The ImPrEP project was made possible, thanks to Unitaid's funding and support. Unitaid accelerates access to innovative health products and lays the foundations for their scale-up by countries and partners. Unitaid is a hosted partnership of the WHO.