Progression of hepatic fibrosis is accelerated in patients co-infected with human immunodeficiency virus and hepatitis C virus compared to hepatitis C virus mono-infected patients. This study aimed to compare ultrasound features and selected clinical and biochemical variables between patients with human immunodeficiency virus/hepatitis C virus co-infection (n=16) versus hepatitis C virus mono-infection (n=16).
MethodsEach patient underwent abdominal ultrasound, and a specific evaluation was performed in order to detect findings consistent with chronic liver disease. Characterization of spleen size, liver structural pattern, diameter of the portal, spleen, and mesenteric veins was based on classical ultrasound parameters. Propensity score was used for control of selection bias and performed using binary logistic regression to generate a score for each patient. The Fisher and Mann–Whitney tests were used to evaluate categorical variables and continuous variables, respectively.
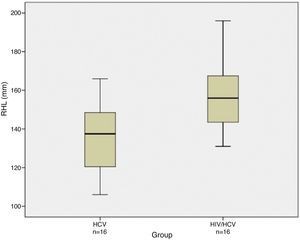
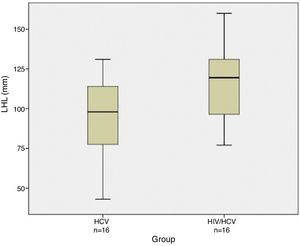
ResultsOn univariate analysis right hepatic lobe size was larger in human immunodeficiency virus/hepatitis C virus patients (157.06±17.56mm) compared to hepatitis C virus mono-infected patients (134.94±16.95mm) (p=0.0011). The left hepatic lobe was also significantly larger in human immunodeficiency virus/hepatitis C virus patients (115.88±22.69mm) versus hepatitis C virus mono-infected patients (95.06±24.18mm) (p=0.0177). Also, there was a strong correlation between hepatomegaly and co-infection (p=0.005).
ConclusionHuman immunodeficiency virus infection was the primary variable influencing liver enlargement in this population. Hepatomegaly on ultrasound was more common among cirrhotic human immunodeficiency virus/hepatitis C virus co-infected patients than among cirrhotic hepatitis C virus mono-infected patients. This aspect is very important in the management of human immunodeficiency virus/hepatitis C virus co-infected patients, because screening for hepatocellular carcinoma is necessary in this population.
Chronic hepatitis is one of the most relevant co-morbidities of patients co-infected with human immunodeficiency virus (HIV) and hepatitis C virus (HCV).1 HIV-infected patients are at increased risk for HCV infection, with an estimated HCV prevalence of 30–35% in this population.1 Among HIV-positive drug users, the reported prevalence of HCV is up to 80%.2,3 Patients co-infected with HIV and HCV develop liver disease more rapidly, and earlier progression to liver cirrhosis has been described in this population.4–7
Liver cirrhosis is usually suspected on the basis of abnormalities in standard liver function, biochemical (blood) tests, non-invasive methods of evaluating fibrosis, and ultrasound (US) examination. The role of radiology in the evaluation of liver cirrhosis is primarily to characterize the morphologic manifestations of the disease, evaluate hepatic and extra-hepatic vasculature, assess the effects of portal hypertension, and detect liver tumors.
There are scarce data in the literature regarding US studies in HIV/HCV co-infected patients, particularly those with liver cirrhosis. US findings could influence the management of these patients in terms of identifying indications for liver biopsy, treatment, and surveillance for hepatocellular carcinoma.
The aim of this case–control study was to compare US features and selected clinical and biochemical variables among HIV/HCV co-infected versus HCV mono-infected patients with hepatic cirrhosis matched for age, gender, and body mass index (BMI). We aimed to answer two questions: (1) Is there any difference in liver size between co-infected and mono-infected cirrhotic patients? (2) Are there demographic and clinical variables related to liver size?
MethodsStudy populationThe initial study population was 45 patients, including 28 HIV/HCV co-infected and 17 HCV mono-infected patients. We applied a propensity score using three demographic characteristics (age, gender, and BMI) and identified 16 patients with HIV/HCV co-infection (study group) and 16 patients with HCV mono-infection (control group). The co-infected patients were selected among patients seeking regular care at the AIDS Outpatient Clinic of the Hospital das Clínicas, Medical School, Universidade de São Paulo. HCV mono-infected patients were selected from those registered at the Department of Gastroenterology, Universidade de São Paulo School of Medicine. Patients were selected from January 2006 to January 2007.
All patients had a diagnosis of HCV infection (seropositive for HCV-RNA) and a histopathological diagnosis of liver cirrhosis. Each patient underwent abdominal US, and a specific evaluation was performed in order to detect findings consistent with chronic liver disease (liver and spleen size, liver texture, diameter of the portal, splenic, and superior mesenteric veins). We analyzed the following additional variables: history of high ethanol consumption (daily intake of more than 60g for females or more than 80g for males for more than 10 years), HCV genotype, US parameters, and the presence of steatosis on liver biopsy.
Patients were excluded if they had a history of chronic liver disease from other etiology (including hepatitis B virus co-infection), opportunistic infections, or other serious medical conditions.
Liver biopsyLiver biopsy was indicated in all subjects to evaluate the severity of hepatic disease. The decision to proceed with a liver biopsy was made during routine work-up for chronic hepatitis C, and was in accordance with accepted clinical practice by physicians staffing the Department of Gastroenterology and Infectious Diseases at the University of São Paulo School of Medicine. Steatosis on liver biopsies was graded according to the following categories: 0 (minimal, <5%), 1 (mild, 5–33%), 2 (moderate, 34–66%), or 3 (severe, >66%). Biopsy was performed at a mean of 12 (standard deviation [SD] 3.6) months after US examination.
Ultrasound examinationAll patients underwent US of the abdomen. The operator was an experienced US examiner and was blinded to other patient variables. Patients were in the supine position for all examinations. For better access to the liver, patients were instructed to raise their hands behind the head, thus increasing the intercostal spaces and the distance from the lower costal margin to the iliac crest. US examination was performed during deep inspiration and with a relaxed abdominal wall. In each case, the liver was examined and visualized in three planes: longitudinal, cross-sectional, and diagonal. Patients fasted for an average of 6h prior to US.
A specific protocol was performed to evaluate the characteristics consistent with chronic liver disease (liver and spleen size, liver texture, diameter of the portal, splenic, and mesenteric veins). The right hepatic lobe size was measured in accordance to prior research: the cranio-caudal diameter was determined in the conventional section in the mid-clavicular line, by measuring from the hepatic dome to the inferior hepatic tip.8–10 The left hepatic lobe was measured at the median line of the abdomen, parallel to the aorta.
Normal liver size was defined as follows: in men with BMI between 22 and 26kg/m2 and women with BMI between 22 and 25kg/m2, normal liver size was 13.5±1.7cm; in men with BMI>26kg/m2 and women with BMI>25kg/m2, normal liver size was 14.5±1.7cm.10 Characterization of other features (spleen size, liver structural pattern, diameter of the portal, spleen, and mesenteric veins) was based on classical US parameters.11
Portal hypertension was defined by the following criteria: (1) portal vein diameter larger than 12mm; (2) mesenteric and splenic veins larger than 9mm; (3) the presence of collateral routes; and (4) spleen index larger than 20cm2 [9]. Liver steatosis was defined on US by the observation of a bright liver echo pattern or by the loss of portal venous walls.12
Clinical and viral variablesMedical records were retrospectively reviewed to ascertain demographic and clinical characteristics, and to obtain laboratory data preceding the US examination. An electronic and written database containing information on all patients was used as the source of laboratory information. On the day of the US examination, measurements of body weight and height were performed in a standardized fashion by clinical research coordinators.
The local Ethics Committee reviewed and approved this study. All patients who participated in the study provided informed consent.
Statistical analysisPropensity score was used for control of selection bias and performed using binary logistic regression to generate a score for each patient (study cases and controls). Variables included in the propensity model were age, gender, and BMI. The Fisher Exact Test was used to verify associations between categorical variables. The Mann–Whitney test was used to evaluate continuous variables. p-Values <0.05 were considered statistically significant.
ResultsMost patients were male (14 in each group [87.5%]). Mean age was 45.19±6.48 years in the co-infected group and 48.13±4.44 years in the mono-infected group (p=0.1453).
On univariate analysis, right hepatic lobe size was larger in HIV/HCV co-infected cirrhotic patients (157.06±17.56mm) compared to HCV mono-infected cirrhotic patients (134.94±16.95mm) (p=0.0011). The left hepatic lobe was also significantly larger in HIV/HVC co-infected patients (115.88±22.69mm) versus HCV mono-infected patients (95.06±24.18mm) (p=0.0177). Hepatomegaly on US was more common among HIV/HCV co-infected patients compared to HCV mono-infected patients. Figs. 1 and 2 show box plots of the right and left hepatic lobes size, respectively, in the mono- and co-infected groups.
To categorize hepatomegaly we adopted a cut-off for the right lobe of 152mm, as described by Kratzer et al.10 We then used the Fisher Exact Test and found a significant correlation between hepatomegaly and co-infection (p=0.005). We found no association between hepatomegaly and HCV genotype 3 versus non-3 (p>0.999).
On univariate analysis, there was no significant difference in the diameter of portal (p=0.783), superior mesenteric (p=0.5079), or splenic veins (p=0.5578) between groups. Spleen size was also similar in both groups (p=0.6978).
Table 1 summarizes US liver steatosis grades of HIV/HCV co-infected patients versus HCV mono-infected patients. There was no difference between groups in terms of US findings of steatosis.
Demographic, ultrasonographic, and histologic characteristics of the study population.
| HIV/HCV | HCV | p-value | |
|---|---|---|---|
| Age (years) | 45.19±6.48 | 48.13±4.44 | 0.145 |
| Male gender (%) | 87.5 | 87.5 | >0.999 |
| BMI (kg/m2) | 23.85±3.40 | 25.73±3.91 | 0.156 |
| High ethanol consumption (%) | 45.45 | 31.25 | 0.686 |
| HCV genotype 3 (%) | 58.33 | 18.75 | 0.049 |
| HCV genotype 1 (%) | 41.66 | 68.75 | |
| HCV genotype 2 (%) | 0 | 12.5 | |
| Right hepatic lobe (mm) | 157.06±17.56 | 134.94±16.95 | 0.001 |
| Left hepatic lobe (mm) | 115.88±22.69 | 95.06±24.18 | 0.017 |
| Steatosis grade on ultrasound | |||
| Mild (%) | 6.25 | 12.5 | >0.999 |
| Moderate (%) | 6.25 | 6.25 | |
| High (%) | 18.75 | 12.5 | |
| Steatosis grade on liver histology | |||
| 0 (%) | 50.0 | 37.5 | 0.697 |
| 1 (%) | 31.25 | 37.5 | |
| 2 (%) | 6.25 | 18.75 | |
| 3 (%) | 12.5 | 6.25 | |
BMI, body mass index.
All patients had a histopathological diagnosis of cirrhosis. There was no significant difference between groups in the degree of steatosis by histology (p>0.05).
On univariate analysis, there was no significant difference between groups according to ethanol consumption.
DiscussionLiver enlargement has been previously observed on abdominal US in HIV-infected patients.13–15 We performed the current investigation to determine if HIV and HCV co-infection produces different pathologic findings. We found that some patients with HIV/HCV co-infection had larger livers than mono-infected patients, even in the presence of established liver cirrhosis. This is a notable finding, as most patients with cirrhosis have smaller livers than non-cirrhotic patients with liver disease. This may be an important feature in evaluating these patients for treatment and surveillance. The main goal of this study was to determine if HIV/HCV co-infected patients had enlarged livers compared to HCV mono-infected patients. To evaluate this hypothesis we designed a case–control study including 16 patients with HIV/HCV co-infection and 16 patients with HCV mono-infection, with groups paired using propensity score. The two groups were similar according to age, gender, and BMI. We analyzed some demographic, clinical, and US variables, including right and left hepatic lobe size, spleen size, and portal, superior mesenteric, and splenic vein measurement on US examination, ethanol consumption, HCV genotype, and grade of steatosis on liver biopsy. Patients were excluded if they had a history of chronic liver disease from another etiology (including hepatitis B virus co-infection), opportunistic infections, or other serious medical conditions.
We found that co-infected patients had significantly larger livers than mono-infected patients on univariate analysis. To determine the influence of other factors on liver size, we analyzed demographic and clinical variables; on univariate analysis there was no significant association between liver size and ethanol consumption, liver steatosis, and HCV genotype. Many other factors may influence liver size in these patients; it was not the objective of this study to identify all significant features related to liver size, but we did confirm that the presence of HIV infection was the main difference between the two groups.
Fat infiltration of the liver can cause hepatomegaly.16 In our patient population, steatosis might play a role in liver enlargement. HCV itself can result in fat deposition in the liver17 and in patients with chronic hepatitis C, steatosis is associated with genotype 3, probably by promoting the production of lipid-rich VLDL that facilitates maturation of HCV precursors by optimizing HCV replication and thus contributing to steatosis.18–21 We did not find a significant association between hepatomegaly and HCV genotype 3. Liver steatosis was not more common in patients with larger hepatic size, thus we could not identify an association between HCV genotype 3 and hepatomegaly in the co-infected group. The presence of HIV infection was the main factor we found differentiating smaller versus larger cirrhotic livers.
The etiology of steatosis in patients with HIV-HCV co-infection is indeed multifactorial. Therefore, we speculate that HIV-infected patients with chronic hepatitis C caused by HCV-3 who are taking antiretroviral therapy may be particularly prone to developing liver steatosis and more severe liver fibrosis. The rising rates of diabetes and obesity among HIV-infected patients22,23 similar to the general US population, may also contribute to non-alcoholic fatty liver disease (NAFLD) in this patient group. In addition, studies suggest that gut-derived lipopolysaccharides may promote hepatic damage.24
Ethanol ingestion and the use of antiretroviral drugs (particularly nucleoside analogs) are often reported to cause liver steatosis.25 HIV is associated with changes in lipid metabolism, as well as with the development of metabolic syndrome with lipoatrophy or lipodystrophy, dyslipidemia, peripheral insulin resistance, and increased hepatic steatosis.26
When we evaluated right hepatic lobe size, measured at the mid-clavicular line, we found co-infected patients to have larger sizes compared to mono-infected patients (p=0.0011). A recent study of age-related changes in abdominal organ structure and function with computed tomography and positron emission tomography showed that the liver enlarges in childhood, but we clearly see a continued size increase up to 49 years of age, when the organ volume achieves 1606mL (18–49 years) and then diminishes to 1467mL (50–81 years).27 We did not evaluate the effect of age on hepatic size because we used age-matched controls for comparison.
In general, diagnostic imaging techniques are claimed to be superior to clinical examination in determining liver size.28,29 Nevertheless, there is a paucity of data regarding normal and borderline values, and no uniform procedure for measuring the size of the liver has been established that can serve as a guideline for US examination.30 In a study published by Gosink and Leymaster,30 data regarding liver specimens obtained at autopsy correlated with anthropometric data of the same patient, and this correlation was used to diagnose hepatomegaly.
It must be highlighted that the estimation of liver size on the basis of a single parameter, such as liver diameter in the right mid clavicular line, is limited, considering the range of liver morphotypes.31 Obesity, accumulation of abdominal gas, and uncooperative patients (lack of coordinative respiration) are other known limitations of this method. Despite these facts, the evaluation of hepatic size is a frequent issue in abdominal US (especially for the determination of hepatomegaly), and this method complements physical examination.29 When we evaluated the left lobe, we performed the same analysis as with the right lobe, with similar results. The common finding of left lobe enlargement in liver disease helps to overcome the limitations of clinical examination, although left lobe measurement is often inaccurately performed and is not standardized for routine US.
To the best of our knowledge, the present study is the first to report enlargement of cirrhotic livers in HIV/HCV co-infected patients. In general, the most common US features in patients with cirrhosis are the presence of irregularity (irregular surface and liver nodules)32 and liver atrophy. In more advanced stages of liver disease, the liver becomes small with a multinodular surface, decreased portal blood flow can be observed by Doppler, and eventually ascites can be detected. Unlike patients with cirrhosis of viral origin, patients with cirrhosis of alcoholic origin and non-alcoholic fatty liver disease may have hepatomegaly on clinical or imaging evaluation.33,34
The finding that hepatomegaly was a common US feature among cirrhotic HIV/HCV co-infected patients may have important implications. In clinical practice, US is currently used to predict the presence of cirrhosis in two general ways. The first is by determining the presence or absence of portal hypertension, and the second is by examining the size, lobar ratios, echogenicity, and echotexture of the liver.32 Although the signs of portal hypertension are usually recognized, in the absence of other clinical or image abnormalities suggestive of cirrhosis, isolated liver enlargement may be misdiagnosed by clinicians as evidence of other clinical conditions related to HIV infection and unrelated to cirrhotic liver disease. Under these circumstances, diagnostic and therapeutic procedures related to the specific liver condition may be postponed, with inevitable consequences to the patient.
This study had some limitations. First, liver biopsies were not performed at the same time as the US examinations. Second, laboratory data concerning serum triglycerides, total cholesterol, high density lipoprotein, and fasting glucose levels were not always collected during the 30 days preceding the US examination or liver biopsy. Therefore, we could not evaluate the association between serum metabolic and non-invasive fibrosis evaluation tests and liver enlargement. These limitations may have contributed to the fact that no association was observed between liver enlargement and presence of liver steatosis on biopsy.
ConclusionsIn conclusion, the presence of HIV infection was the primary variable influencing liver enlargement in this population. Hepatomegaly was a more common US feature among cirrhotic HIV/HCV co-infected patients than among cirrhotic HCV mono-infected patients. This aspect could be important in the management of HIV/HCV co-infected patients, because screening for hepatocellular carcinoma is necessary in this population.
Conflict of interestAll authors declare to have no conflict of interest.
We are deeply indebted to the Alves de Queiroz Family Fund for the continuous sponsoring support.