Epstein-Barr virus has been recently associated with the onset of multiple sclerosis, yet understanding how it elicits autoimmunity remains elusive. We investigated the relation between Epstein-Barr virus reactivation and disease development in different subtypes of multiple sclerosis.
MethodsIn the present research, we have determined the Epstein-Barr virus-DNA load by quantitative real-time polymerase chain reaction and Epstein-Barr virus antibody levels by EIA technique in both multiple sclerosis patients (n=78) and healthy controls (n=123).
ResultsOur results demonstrated increased titer of both anti-Epstein-Barr virus-IgG and IgM antibodies in patients (91.02% vs 82.11% in controls, p<0.001 and 14.1% vs 4.06% in controls, p<0.001, respectively). Overall, Epstein-Barr virus reactivation was found in 68.75% of subtypes of multiple sclerosis, 4.54% of multiple sclerosis primary subtype, and in only 3.25% of healthy control subjects. Moreover, in samples of patients with disease relapse (exacerbation) cell free viral DNA was elevated in contrast to other patients (p<0.001).
ConclusionsThese findings provide further support for the detrimental effects of Epstein-Barr virus in the reactivation of multiple sclerosis attacks.
Multiple sclerosis (MS) is the most prevalent demyelinating disease among young adults, affecting many people in the developing countries.1 There are several different forms of MS. In some cases, symptoms are present all the time and get progressively worse. In other cases, the symptoms tend to come and go in periods of remissions and exacerbations (flares, relapses or attacks).2 An important step in managing this condition is identifying the factors that cause MS exacerbations, and then taking actions to minimize exposure.3 No virus has been definitively implicated as a causative factor for MS, but certain Human Herpes Viruses (HHVs) have been linked with the development of MS.4,5 There is strong epidemiologic evidence linking MS to infection with the B-lymphotropic γ-herpesvirus Epstein-Barr virus (EBV).6,7 However, the underlying mechanisms currently remain elusive. One hypothesis is that EBV or EBV-infected B cells might directly infiltrate the CNS, eliciting an EBV-specific immune response which subsequently leads to damage of surrounding tissue.8,9 It is also proposed that deprivation of sunlight and vitamin D at higher latitudes facilitates the development of MS.10 Several hypotheses have been addressed to explain the breaking of immune tolerance by EBV, including molecular mimicry between viral and myelin components,11–13 EBV-induced expansion of auto-reactive B cells,14 and induction of heat-shock proteins and super-antigens,15 but evidence that these mechanisms are relevant to MS is not available yet. Because of its ability to establish a latent infection in B cells, to promote their proliferation and activation, and to reactivate periodically providing a constant antigenic challenge to the immune system, EBV is well suited to be a trigger of chronic inflammatory states and exacerbation in MS.16,17 In this case-control study we attempted to determine the seroprevalence of anti-EBV antibodies (IgG and IgM) and distribution of EBV-DNA in various specimens to determine the role of systemic active EBV infection in pathogenesis of MS.
Materials and methodsPatients and samplesThe study, approved by the Zahedan University of Medical Science Multiple Institutional Review Board, was conducted with all clinical samples from MS patients who were treated at the Department of Neurology, Ali-ebn Abitaleb Hospital, Zahedan, Iran, and also, Healthy Blood Donors (HBD) who voluntarily agreed to participate in this research at the central medical laboratory of Zahedan from December 2008 through July 2010. MS patients (in southeast of Iran) were diagnosed with Magnetic Resonance Imaging (MRI) and McDonald criteria were collected.18 We analyzed 201 different samples; 78 patients and 123 people as the healthy control group. The patient group comprised 22 men (mean age, 28.8 years; age range, 17–48 years) and 56 women (mean age, 30.3 years; age range, 16–52 years). The control group of HBD comprised 34 men (mean age, 26.4 years; age range, 17–42 years) and 89 women (mean age, 26.0 years; age range, 17–50 years).19 EDSS score for all patients at the time of inclusion were below scale 5.0, except of three individuals with secondary progressive MS (SPMS) [scale 6.5] and five with relapsing remitting multiple sclerosis (RRMS) [scale 5.0]. All patients had at least one annual relapse, during two years before inclusion in the study. Serum, PBMCs and unstimulated whole saliva samples were collected by standard methods. A total of 38 CSF samples (1.5mL) were collected from MS patients (RRMS=22, SPMS=6, primary progressive multiple sclerosis – PPMS=10) after lumbar puncture (LP) in sterile containers and were centrifuged for 15min at 180g at 20oC to obtain cell-free supernatants. Serum samples from 11 patients with RRMS and six patients with SRMS (17 samples in total) were obtained during periods of disease exacerbation and the relation was tested between defined EBV reactivation periods and exacerbation rate for a mean of one year. All Specimens were stored at −70°C until the experiment was performed. Multiple specimens were available for each patient, and all of them were tested. When possible, clinical materials were tested more than once.
DNA extraction and quantitative real-time PCR (qPCR)EBV DNA extraction was performed for 100μL of samples using RIBO-prep nucleic acid extraction kit (Interlabservice, Moscow, Russia) according to the manufacturer's protocol. Real-time PCR was performed using the AmpliSens EBV-screen-FRT kit (Inter lab service) according to the manufacturer's protocol. This real-time PCR assay showed to be sensitive, specific, and reproducible. The assay has an internal control, which allows inefficient extraction or PCR inhibition to be detected. Real-time amplification was carried out using 10μL DNA eluate combined with 10μL PCR-mix-1-FL and 5μL PCR-mix-2-FL using Rotor-Gene 3000 Instrument (Corbett Research, Sydney, Australia) with the following cycling parameters: pre-denaturation at 95°C for 15min, 95°C for 5s, 60°C for 20s and 72°C for 15s for 45 cycles. Data acquisition was performed in both JOE/HEX/Yellow channel for EBV DNA and in the FAM/Green channel for β-Globin gene DNA during the annealing (60°C) stage. For quantification of EBV DNA two standard positive samples of KSG1 (104 copies per reaction mixture) and KSG2 (102 copies per reaction mixture) were included in the run (Interlabservice). Calculations of Ct, preparation of standard curve and quantification of DNA in each sample were performed by Rotor-Gene Operating Software, version 1.8 (Corbett Research).
EBV antibody responseConcentrations of serum EBV IgG VCA and IgM VCA were measured based on EIA method in an automated instrument, according to the manufacturer's instructions (Biotrin, The Rise, Mount Merrion, Co., Dublin, Ireland).
Viral reactivation markersIn this study, we considered reactive EBV infection, when IgG and IgM were positive by immunoassay, and/or two or more consecutive positive qPCR, and/or load EBV≥200 copies in serum, or ≥150 copies in both saliva and PBMNCs.
Statistical considerationsThe Statistical Package for Social Sciences (SPSS Inc., Chicago, IL, USA), Version 16 was used for statistical analysis. χ2 analysis was applied to analyze categorical variables, t tests for continuous variables and Mann–Whitney U tests for non-parametric variables. The nominal variable groups were compared by Pearson's correlation coefficient. All p-values are two-tailed and significant at p<0.05 or p<0.01 depending on the statistical method. Relative risk was calculated using the Word Processing, Database, and Statistic Program for Public Health Epi Info 6, Version 6.04B [Centers for Disease Control and Prevention (CDC), USA, World Health Organization, Geneva, Switzerland].
Ethical considerationsThe study conformed to the Helsinki declaration and was reviewed and approved by the local research committee; written informed consent was obtained from all subjects.
ResultsDetection of IgG and IgM antibodies against EBV-VCARecent studies have demonstrated that at least 91.02% of MS patients are positive for EBV- specific IgG (IgG+) antibodies in contrast with 82.11% of healthy controls (Table 1). 100% of SPMS patients were IgG+ in their serum samples compared to 93.47% of the RRMS, and 80.95% of PPMS samples (Table 2). The detection of anti-EBV IgM from healthy volunteer donors and MS patients, independent of EBV-DNA detection in PBMCs, is indicative only of a new infection and found in 3.25% of controls but in none of the patients (p<0.05). Moreover, among MS subtypes, only SPMS (36.36%) and RRMS (15.21%) patients showed anti-EBV-IgM as a sign of reactivation in their serum in contrast with PPMS (p<0.001). On the other hand, patients had higher concentration of both IgM and IgG compared to controls (Table 1).
Prevalence of EBV-DNA (copies/mL) and EBV-antibodies (U/mL) among controls and MS patients. EBV-DNA was analyzed via qPCR as described previously. Concentration of plasma anti-EBV, IgG and IgM were measurement in an automated instrument, according to the manufacturer's instructions. Data are representative of three independent experiments.
| Patients (n=78) | Controls (n=123) | Sig. (2-tailed) | |
|---|---|---|---|
| P/N (%) [mean±SD] | |||
| Anti-IgG (U/mL) | 71/7(91.02) [20.26±6.67] | 101/22(82.11) [15.06±4.22] | P=0.001 |
| Anti-IgM (U/mL) | 11/67(14.10) [31.24±3.73] | 5/118(4.06) [24.70±2.14] | P=0.001 |
| Saliva-DNA (copies/mL) | 39/39(50) [134±18.75] | 47/76(38.21) [158±41.18] | P=0.001 |
| Serum-DNA (copies/mL) | 30/48(38.46) [289±62.16] | 21/102(17.07) [274±41.87] | NS |
| PBMCs-DNA (copies/mL) | 53/25(67.94) [160±52.04] | 51/72(41.46) [155±29.91] | NS |
PBMCs, peripheral blood mononuclear cells; CSF, cerebrospinal fluid; P, positive; N, negative; NS, not significant.
Prevalence of EBV-DNA (copies/mL) and EBV-antibodies (U/mL) among different subtypes of MS. EBV-DNA was analyzed by qPCR as described previously. Concentration of plasma anti-EBV, IgG and IgM were measurement in an automated instrument, according to the manufacturer's instructions. Data are representative of three independent experiments.
| Saliva | Serum | PBMCs | CSF | Anti-IgG | Anti-IgM | |
|---|---|---|---|---|---|---|
| P/N (%) [mean±SD] | ||||||
| MS (n=78) | ||||||
| 1RRMS (n=46) | 28/18 (60.86) | 21/25 (45.65) | 37/9 (80.43) | 7/15 (31.81) | 43/3 (93.47) | 7/39 (15.21) |
| CSF (n=22) | [131±14.27] | [288±46.90] | [160±53.29] | [141±20.81] | [21.37±6.51] | [31.68±4.09] |
| 2SPMS (n=11) | 9/2 (81.81) | 8/3 (72.72) | 10/1 (90.90) | 4/2 (66.66) | 11/0 (100) | 4/7 (36.36) |
| CSF (n=6) | [144±28.40] | [307±85.17] | [185±51.25] | [131±10.00] | [24.60±6.38] | [30.47±3.41] |
| 3PPMS (n=21) | 2/19 (9.52) | 1/20 (4.76) | 6/15 (28.57) | 0/10 (–) | 17/4 (80.95) | 0/21 (–) |
| CSF (n=10) | [128±7.07] | [168] | [122±7.50] | [–] | [14.62±2.82] | [–] |
| Sig. (2-tailed) | ||||||
| Subtypes(1,2) | NK | NS | NS | NS | NS | NS |
| Subtypes(1,3) | NS | P<0.05 | P<0.01 | – | P<0.001 | – |
| Subtypes(2,3) | NS | P<0.001 | P<0.05 | – | P<0.001 | – |
PRMCs, peripheral mononuclear cells; CSF, cerebrospinal fluid; PPMS, primary progressive MS; RRMS, relapsing-remitting MS; SPMS, secondary progressive MS; P, positive; N, negative; NS, not significant.
EBV DNA load in serum samples did not differed between MS patients and controls (Table 1). In the saliva samples, 50% of patients were EBV+ compared to 38.21% of the controls (Table 1). Viral DNA was found in all saliva samples that previously were positive for viral DNA in their PBMCs both in patients and controls. Saliva showed much higher prevalence of viral sequence than serum samples in controls (p=0.001). In the PBMCs samples, 67.94% of patients were EBV+ in contrast to 41.46% of the controls (Table 1). EBV DNA was detected only in seven CSF samples of RRMS (31.81%) and four CSF samples of SPMS (66.66%) obtained during an exacerbation but were not found in CSF of patients with remission or patients with PPMS (Table 2). As shown in Table 2, 80.43% of patients with RRMS, 90.9% of patients with SRMS and 28.57% patients with PPMS had EBV sequences (EBV+) in PBMCs. Six patients with RRMS (13.046%) and four patients with SRMS (36.36%) showed further positivity in all specimens (Table 2). Furthermore, among patients’ samples, 11 (14.1%) individuals showed positive results in all specimens in contrast to none of the controls (Table 2).
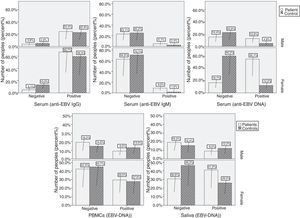
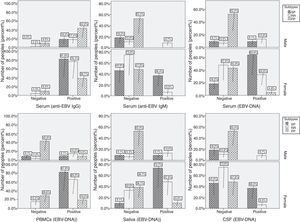
Systemic infection and disease exacerbationSystemic EBV infections were found in 68.75% of patients and in only 4.54% of controls (p<0.001). As a measure of reactivation, combined qPCR results and IgG serology showed that 16.66% of the patients had reactive EBV infections compared to 3.25% of controls. Reactive viral infection in these patients was confirmed by the detection of specific anti-EBV IgM antibodies in their serum. Viral DNA in serum and specific IgM antibodies in plasma were not detected in 82.11% of healthy controls (101/123) and 48.71% of patients (38/78). A strong association was found between EBV reactivation and MS attacks when MS primary stages (4.54%) were compared to other stages (p<0.001). Neither viral DNA in serum, nor the presence of IgM specific antibodies, or elevated titers of IgG antibodies to EBV were found in 8.69% of RRMS (4/46), 18.18% of SPMM (2/11) and 42.85% of PPMS (9/21), confirming that in these patients EBV infection remained latent. Episodes of defined EBV reactivation were observed in a subgroup (11 patients with RRMS and six patients with SRMS), and these episodes were associated with increased relative risk (RR) for disease exacerbation. In these subgroup of patients, the annual number of reactivation was 3.1 in the group of 11 patients who had one or more relapses, compared to 1.12 in the group of six patients who did not experience a relapse (p<0.05). In a 4-week period beginning two weeks before reactivation and ending two weeks after reactivation, the RR of relapse was 3.5 (p<0.05) compared to all other periods. Furthermore, all patients with disease exacerbation showed reactivated infection and EBV-DNA in their CSF samples. Prevalence of EBV-DNA and anti-EBV antibodies was demonstrated by a comprehensive analysis performed among males and females in both control and patient groups (Figs. 1 and 2). In all cases, female patients showed higher positivity (Fig. 1) and systemic EBV infection was more prevalent among females compared to males (p<0.001). Female patients with SPMS showed higher prevalence of EBV-DNA and anti-EBV antibodies compared to both males and other MS subtypes (Fig. 2). Increased EBV-DNA concentrations tended to be associated with EBV systemic infection, but associations with additional components such as MS subtypes and gender were even stronger.
Significant difference and positive correlation with concentration of EBV-DNA in saliva and EBV-DNA in serum were found in both groups (p<0.005), but a significant inverse correlation with EBV-IgG and IgM response was found only in the patient group (Tables 3 and 4). No correlation was found between detection of EBV-DNA in CSF and detection of EBV-DNA in other samples, or with EBV-IgG and IgM concentrations (Table 3). Serologically, immune status showed poor correlation with IgG concentration and detection of EBV-DNA in serum for both groups (p<0.005). There were no statistically significant correlations between detection of EBV-DNA in serum and EBV-DNA in PBMCs in both groups (Tables 3 and 4). There was direct correlation between EBV-IgG concentration and detection of EBV-DNA in PBMCs in both groups (Tables 3 and 4). Again, a positive correlation was observed between EBV-DNA in saliva and increased EBV-IgG concentration only among patients (Table 3). We found a positive correlation between the detectability of EBV-DNA in CFS from patients and exacerbation, as well as a decrease in EBV IgG/IgM ratio.
Correlation of EBV-DNA detection in different specimens (EBV+) with EBV seroprevalence in MS patients.
| Correlations in MS patients | ||||||
|---|---|---|---|---|---|---|
| IgG | IgM | Serum | PBMCs | Saliva | CSF | |
| EBV-IgG (U/mL) | ||||||
| Pearson correlation | 1 | −.412 | .437* | .580** | .308 | .244 |
| Sig. (2-tailed) | .209 | .016 | .000 | .057 | .469 | |
| EBV-IgM (U/mL) | ||||||
| Pearson correlation | −.412 | 1 | −.242 | −.286 | −.217 | −.308 |
| Sig. (2-tailed) | .209 | .474 | .393 | .521 | .387 | |
| Serum DNA (copies/mL) | ||||||
| Pearson correlation | .437* | −.242 | 1 | .211 | .436* | .121 |
| Sig. (2-tailed) | .016 | .474 | .264 | .026 | .723 | |
| PBMCs DNA (copies/mL) | ||||||
| Pearson correlation | .580** | −.286 | .211 | 1 | .146 | .314 |
| Sig. (2-tailed) | .000 | .393 | .264 | .380 | .346 | |
| Saliva DNA (copies/mL) | ||||||
| Pearson correlation | .308 | −.217 | .436* | .146 | 1 | −.248 |
| Sig. (2-tailed) | .057 | .521 | .026 | .380 | .463 | |
| CSF DNA (copies/mL) | ||||||
| Pearson correlation | .244 | −.308 | .121 | .314 | −.248 | 1 |
| Sig. (2-tailed) | .469 | .387 | .723 | .346 | .463 | |
Correlation of EBV-DNA detection in separate specimens (EBV+) with EBV seroprevalence in healthy controls.
| Correlations in healthy controls | |||||
|---|---|---|---|---|---|
| IgG | IgM | Serum | PBMCs | Saliva | |
| EBV-IgG (U/mL) | |||||
| Pearson correlation | 1 | .457 | .300 | .570** | .562** |
| Sig. (2-tailed) | .543 | .199 | .000 | .000 | |
| EBV-IgM (U/mL) | |||||
| Pearson correlation | .457 | 1 | −.083 | −.940 | −.343 |
| Sig. (2-tailed) | .543 | .917 | .060 | .572 | |
| Serum DNA (copies/mL) | |||||
| Pearson correlation | .300 | −.083 | 1 | .216 | .549** |
| Sig. (2-tailed) | .199 | .917 | .346 | .012 | |
| PBMCs DNA (copies/mL) | |||||
| Pearson correlation | .570** | −.940 | .216 | 1 | .617** |
| Sig. (2-tailed) | .000 | .060 | .346 | .000 | |
| Saliva DNA (copies/mL) | |||||
| Pearson correlation | .562** | −.343 | .549* | .617** | 1 |
| Sig. (2-tailed) | .000 | .572 | .012 | .000 | |
A viral trigger involved in MS has been suggested more than 100 years ago,20 and an extensive list of candidate viruses has emerged since then. Several clinical studies have suggested that MS in general as well as episodes of disease exacerbation are associated with concomitant viral or microbial infections.21–23 Virus may play a role, since MS relapses are often associated with common viral infections.24 Although many infectious microorganisms have been investigated, no organism has emerged as a proven trigger. Different patients may be affected by different organisms, and the infections may cause some, but not all, cases of MS. Organisms that are at the top of the suspect list are those that can affect the central nervous system. The role of EBV in the pathogenesis of MS has been debated in recent years and it has not been clarified whether active EBV infection is specific to MS.25–28 The frequency of EBV specific IgG (measuring latent infection) in normal population was 82.92%, relatively consistent with the average global frequency of 90%.29 However, researchers have discovered that people who are especially sensitive to the virus and have unusually high levels of EBV antibodies may have a greater risk of developing MS.30,31 In recent years, there has been an improved understanding of the epidemiology, pathogenesis, and long-term disabilities associated with EBV infection.32–34 There are evidences that EBV reactivation is associated with clinical disease activity in MS when reactivation is defined as a pattern of increased IgM and IgA levels against EBV.35 Although viral load does not appear to differ between MS patients and healthy EBV-infected controls, the presence of EBV-DNA has been detected more frequently in serial samples of MS patients with high disease activity compared to those with low disease activity.36–38 The major focus of our research was to characterize the extent and distribution of EBV in the pathogenesis of MS. Very little is known about the prevalence of EBV in Iranian MS patients or general population. Analysis of serum EBV-DNA demonstrated that there is a statistically greater likelihood of detecting EBV-DNA in the CSF of SPMS patients than other courses. This study supports the role of EBV in the pathogenesis of MS by suggesting that the presence of systemic EBV infection coincides with developing courses (SPMS and RRMS). We suggest that there may be multiple ‘triggers’ by which foreign antigens, including infectious agents, may be associated with immune attacks on the CNS. We also propose that EBV may be one such trigger and if so, the mechanism(s) by which this virus is associated with the pathogenesis of MS will be important to define. Salivary glands are a potential site for EBV persistence and saliva is a vehicle for transmission of the virus, either from mother to child or between children. EBV-DNA detection in PBMC and salivary glands has no clinical relevance because the virus can be latent in them and its presence does not discriminate between active infection and latent stages. SPMS patients had significantly higher levels of serum EBV IgM compared to other patients. Increased IgM antibodies beside systemic infection could represent EBV reactivation and would be consistent with the hypothesis that this virus may be linked with MS pathogenesis. These results agree with the finding of researchers who reported a higher positivity for EBV-DNA and antibodies in serum and CSF of MS patients with exacerbation.39–45 We emphasize that only through well-controlled intervention clinical trials with effective and safe antiviral can a causal role be of any infectious agent in MS could be tested. In conclusion, high levels of EBV-DNA have been detected in serum, saliva and CSF of MS patients with exacerbation, as well as in their PBMCs. Due to the high prevalence of latently infected individuals in the healthy population, it was difficult to establish a causative role of EBV in this disease. The majority of healthy subjects are seropositive for the virus, and studies showed high reactivation of EBV in patients with RRMS and SPMS. Recently, it was shown that 56.41% of PBMCs from MS patients harbor EBV-DNA in a latent, nonproductive form, which dramatically differ from the control population (35.77%). Therefore, to establish a correlation, it is necessary to discriminate between latent and productive infections. The association of EBV with MS remains controversial and a more extensive understanding of EBV neurotropism and its association with the disease process is required.
ConclusionsThe reactivation of EBV infection in MS patients was supported by serologic findings and molecular detection. As prevalence of anti-EBV IgG in serum and EBV-DNA in PBMCs in both patient and control groups was relatively similar, we concluded that both patients and controls had active infection previously and recently established latent infection. Alternatively, because of high copy number of DNA in serum and also lower titer of anti-EBV IgG in contrast with anti-EBV IgM observed in patients with RRMS and SPMS, we propose that reactivation occurred in this group. On the other hand, the presence of EBV-DNA in CSF samples, which is a sharp marker of reactive viral infection, was detected only in patients with progressive MS and strongly validated our hypothesis. The absence of EBV-DNA in CSF of some patients with active MS may be associated with an early stage of viral replication. Although this study is prospective in design, we cannot definitively prove that EBV plays a causative role in MS.
Conflict of interestAll authors declare to have no conflict of interest.
This research was financially supported by Zahedand's University of Medical Science, Zahedan, Iran. We appreciate Dr. A. Moghtaderi for his helpful efforts in sample collecting.