China’s compulsory annual livestock anthrax vaccination policy has remarkably reduced but not completely eradicated human anthrax infections. Herein we describe a sporadic human cutaneous anthrax outbreak involving two cases in 2018 in Shaanxi Province, both involving herdsman who dealt with unvaccinated and potentially sick cattle. Both patients showed Bacillus anthracis-positive blister smear and blood culture. Treatment with penicillin was followed by uneventful recovery for both. The prompt performance of the prophylactic measures successfully interrupted the further transmission of this sporadic human cutaneous anthrax outbreak.
Anthrax is an acute infectious disease caused by the Gram-positive Bacillus anthracis, spore-forming bacterium. Annual estimates of cases worldwide range from 20,000 to 100,000, and it is still considered a major public health threat in Africa, the Middle East, South America, central Asia and Haiti.1
In China, a total of 3379 human anthrax cases were reported between 2005 and 2014.2 Although these cases involved individuals across all provinces – representing vastly different physiographic features in terms of land cover, altitude, climate, seasonality, animal husbandry practices and topsoil features as well as human density and spatial aggregation – nearly all (97.7%) were similarly of the cutaneous form.2,3 Herein we report two new cases of cutaneous anthrax that occurred in Shaanxi Province in 2018, describing their early diagnosis and successful treatment, which interrupted the further transmission of this infectious disease in the region.
The medical records for the two 2018 cases of cutaneous anthrax in Shaanxi Province were reviewed. In both cases, the diagnosis of anthrax had been based upon the clinical aspect of the cutaneous lesions, epidemiological data (including occupation and history of exposure to potentially sick animals or animal products), and positive microorganism cultures (in both blister discharge and blood).
Patient 1# is a 42-year-old man who was a cattle dealer from the Zhongnan village in the Qinling mountains and was admitted in early September 2018. The patient complained of a painless red lesion on the right forearm that had been followed by development of localized sores and swelling for four days. One day prior to the hospital visit, the patient described experiencing progressive malaise, confusion, and an intense fever (40°C).
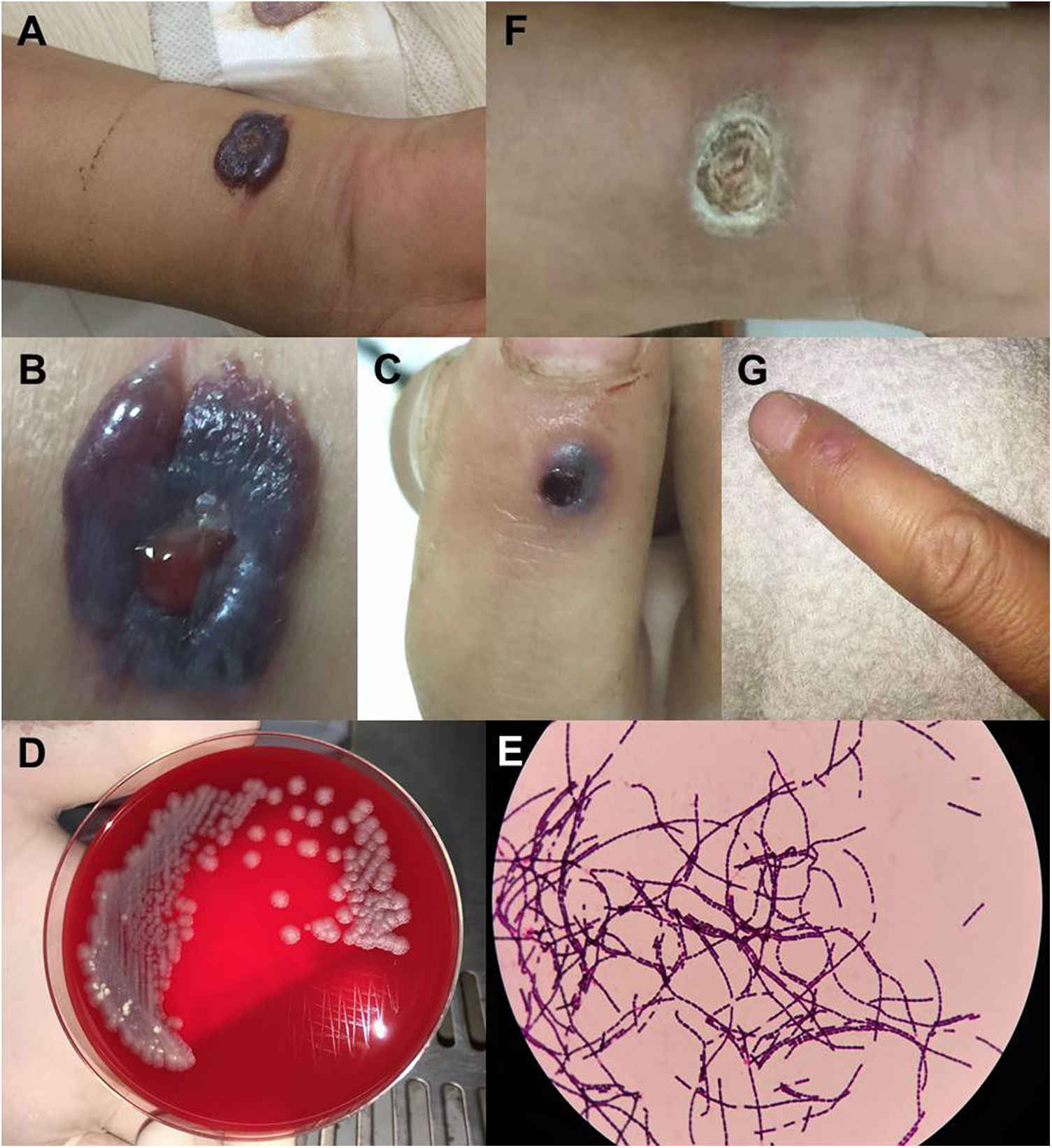
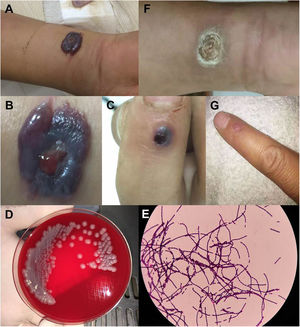
Physical examination revealed severe edema, multiple bullae and a central black hemorrhagic eschar protruding out of the skin (5cm×6cm) on the right forearm (Fig. 1A, B), visible indurated eschar on the dorsal and distal sides of the right index finger (Fig. 1C), and bilateral axillary lymph node enlargement.
Human cutaneous anthrax in Patient 1. A–B) Severe edema on the right forearm, with multiple bullae and a centrally located black hemorrhagic eschar; C) blisters on the dorsal and proximal sides of the index finger; D) gray-white rough colonies grown on blood agar medium for 24h after smear of the discharge fluid; E) blood cultures were positive and identified as Bacillus anthracis; F–G) regression of the skin lesion after penicillin treatment.
Laboratory test showed white blood cell count at 11.8×109/L (normal limits 4.5–10×109/L). Cytology smear of discharge from various lesions of the right forearm showed Gram-positive rods in all (Fig. 1D). Culture of both discharge and blood showed positivity for Gram-positive bacteria, further identified as B. anthracis (Fig. 1E). He was diagnosed with cutaneous anthrax and isolated, and received penicillin treatment for 15 days, along with 5mg dexamethasone for five days. His symptoms went into remission (Fig. 1F, G).
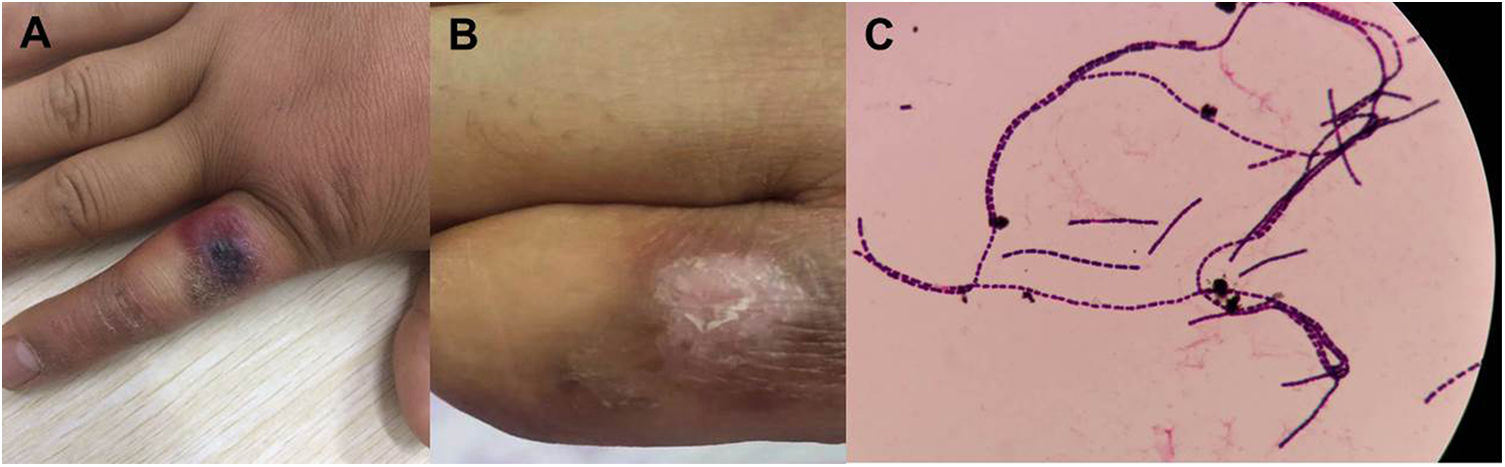
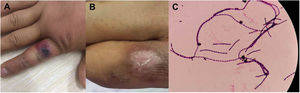
Patient 2# is a 27 year-old man who was a partner of patient 1 and had performed butchering of a cow alongside. He presented to our hospital two days after the first case, complaining of right index finger edema. The patient described having noticed a small, painless red lesion on the right finger three days prior. On examination, there was a 2×1.5cm hemorrhagic bulla, with swollen skin surrounding an indurated eschar on the dorsal and proximal sides of the right index finger (Fig. 2A). Laboratory tests showed normal white blood cell and biochemical parameters. Blood and discharge culture showed positivity for B. anthracis (Fig. 2C). The patient was immediately segregated and prescribed penicillin for 10 days. The swelling improved and the patient recovered uneventfully (Fig. 2B).
In addition to segregating and treating the two patients, our staff immediately traveled to the presumed location of exposure (the slaughterhouse which served three nearby villages, including a farm and several households maintaining free-ranging livestock), where B. anthracis was found on the chopping board. Upon alerting the municipal health bureau and relevant epidemic prevention departments, emergency measures were put into place to prevent spread of this disease. The slaughterhouse underwent rigorous disinfection. Furthermore, the people who consumed the beef from this slaughterhouse were contacted and underwent detailed physical examination. Again, the other three cattle from the patients’ households, which maintained free-ranging livestock, were killed and buried intact. The prompt performance of these prophylactic measures successfully interrupted the further transmission of this infectious disease in the following 10 months.
Anthrax is a primary acute zoonotic infection caused by the Gram-positive, spore-forming bacterium B. anthracis, which exists in two forms: spore and vegetative.3 The spore can resist physical stress and remain viable in soil for decades.3 Although anthrax is a rare disease, inoculation with the bacterium leads to death in 20% of cases when not treated on time.4 Cutaneous anthrax is the least fatal type, being caused mainly by host reactions to the bacterial capsule and toxins.
Human cutaneous anthrax mainly occurs in the body areas directly exposed to the bacterium, commonly being head, neck, and upper extremities. Typical cases begin with a painless carbuncle that rapidly becomes necrotic, possibly influencing the surrounding areas and often being accompanied by inflammation of regional lymph nodes.4 The initial skin lesion is a painless inflammatory papule, with an itchy or burning sensation. Next, blisters or pustules form, being surrounded by rigid non-sag edema. The necrotic lesion subsequently ruptures and a carbonaceous black scab forms, surrounded by satellite lesions, such as blisters and pustules.5,6
Climate is one of the main factors of anthrax outbreak.7 In China, most human anthrax cases have occurred in western regions and incidence peaks in the dry month of August.2 The Qinling mountains retard airflow and inhibit the marine current from the south, making the northern climate of this region dry with especially high temperatures in the summer. The two cases of cutaneous anthrax described herein occurred in late August; at that time of year, the temperature in Qinling is between 22–39°C, with only eight days of light rain or shower (Fig. S1). This climatic condition is suitable for the survival and reproduction of B. anthracis.8
During the period of 2005–2014, 86.7% of human anthrax cases in China involved farmers and herdsmen, and rural cases accounted for 92.4% of all cases.2 Indeed, according to the World Health Organization, cattle are the major source for human anthrax, 4 and the number of cattle slaughtered annually in China reportedly increased from 1980 to 2013. Both our patients infected with anthrax were farmers and cattle dealers, who deal with cattle extensively and have a higher risk of exposure to potentially sick animals.
Immediately after our patients were diagnosed, our staff went to the presumed location of infection and confirmed the presence of B. anthracis. The overall response to the two cases of anthrax – segregating patients and initiating timely treatment, rigorously disinfecting the infectious focus, and destroying any other possibly ill cattle – almost certainly kept others from infection.
The live attenuated Sterne strain is the most widely used vaccine.9 Livestock anthrax vaccination is a proactive approach for preventing the epidemic spread of this disease, which is the most effective method to control anthrax in endemic regions, and when combined with improvements in occupational safety contributes to a notable reduction in incidence among both humans and animals.9,10 In contrast, a study in Georgia indicated that when the country ended its policy of compulsory annual livestock anthrax vaccination, the overall risk of human anthrax increased >5-fold.11 The Chinese government requires mandatory anthrax vaccination of livestock. If economic factors lead to unvaccinated livestock and the slaughter of potentially sick cattle, an anthrax epidemic can occur. It is important for the governmental instrumentalities to continue to conduct quarantined inspection during the purchase, transportation, slaughter and processing of livestock. In addition, the persons who are engaged in purchasing, processing and slaughtering animal products should also be inoculated with the B. anthrax vaccine.
Penicillin is treatment of choice for cutaneous anthrax, followed by aminoglycosides, such as gentamicin, streptomycin, amikacin, etc.12 However, B. anthracis has been reported to be resistant to these antibacterial drugs. Some studies suggest that short-course antibiotic therapy is as effective as standard-duration therapy in cases of uncomplicated cutaneous anthrax, and that steroid therapy may not be necessary, especially for mild cases.12,13 Thus, the World Health Organization has suggested intramuscular procaine penicillin, oral amoxicillin or penicillin V for mild uncomplicated cases of cutaneous anthrax.14 For our two patients, both blood and discharge cultures showed B. anthracis and penicillin sensitivity; indeed, penicillin treatment was followed by uneventful recovery of both. This outcome was achieved in Patient 1# despite the combination of dexamethasone being used (with the intent of rapidly relieving the skin swelling).
ConclusionWe report two cases of sporadic human cutaneous anthrax outbreak involving cattle dealers. The prompt performance of prophylactic measures successfully interrupted the further transmission of this outbreak in the following 10 months. Our report emphasizes the importance of vaccination and of taking measures urgently in preventing anthrax incidence and disease transmission.
Grant supportThis work was supported by Personnel training special funds of the Second Affiliated Hospital of Xi’an Jiaotong University [RC(GG)201501] and the Fundamental Research Funds for the Central Universities (No. xzy012019107).
Conflicts of interestThe authors declare no conflicts of interest.
Author contributionsAll authors collaborated in the study design; Liu YY, Li YQ, Fu JJ, and Ji FP participated in diagnosing and managing these two patients; Liu YY, Wang QX, and Fu JJ participated in epidemiological investigation included slaughterhouse disinfection, physical examination and follow-up of people consuming suspicious cattle; Liu YY, Li YQ, and Ji FP extracted and analyzed the clinical data; Liu YY prepared the first manuscript draft; Fu JJ and Ji FP modified the manuscript subsequently; all authors have reviewed and approved the final manuscript.
We would like to thank Dr. Wei Shi from Shaanxi Provincial Center for Disease Control and Prevention, and Dr. Jifeng Liu and Qian Li from Xi'an Centre for Disease Control and Prevention, for their kind help in carrying out epidemiological investigation. We also would like to thank the patients and suspected exposed populations for their collaboration in conducting epidemiological investigations and performing measures to interrupt the spread of disease.