The antifungal activity of some statins against different fungal species has been reported. Thus, at the first moment, the in vitro antifungal activity of simvastatin, atorvastatin and pravastatin was tested against Candida spp. and Cryptococcus spp. Then, in a second approach, considering that the best results were obtained for simvastatin, this drug was evaluated in combination with antifungal drugs against planktonic growth and tested against biofilms of Candida spp. and Cryptococcus spp. Drug susceptibility testing was performed using the microdilution broth method, as described by the Clinical and Laboratory Standards Institute. The interaction between simvastatin and antifungals against planktonic cells was analyzed by calculating the fractional inhibitory concentration index. Regarding biofilm susceptibility, simvastatin was tested against growing biofilm and mature biofilm of one strain of each tested yeast species. Simvastatin showed inhibitory effect against Candida spp. and Cryptococcus spp. with minimum inhibitory concentration values ranging from 15.6 to 1000mgL−1 and from 62.5 to 1000mgL−1, respectively. The combination of simvastatin with itraconazole and fluconazole showed synergism against Candida spp. and Cryptococcus spp., while the combination of simvastatin with amphotericin B was synergistic only against Cryptococcus spp. Concerning the biofilm assays, simvastatin was able to inhibit both growing biofilm and mature biofilm of Candida spp. and Cryptococcus spp. The present study showed that simvastatin inhibits planktonic cells and biofilms of Candida and Cryptococcus species.
The incidence of invasive fungal infections, especially those caused by opportunistic fungi of the genus Candida and Cryptococcus, has proportionally increased with the increase in the number of hosts with impaired immunity.1–4 In addition, in vitro resistance to antifungal drugs among Candida spp. and Cryptococcus spp. strains recovered from humans and animals has been reported.5–11
This scenario motivates the search for new compounds with antifungal potential. Originally, the first statins were described as metabolites of microorganisms with the ability to lower blood cholesterol.12 Later, it was demonstrated that these compounds reduce the growth of several fungal species,13–15 including the yeasts Candida spp. and Cryptococcus neoformans16 and the filamentous fungi Mucor spp. and Rhizopus spp.17 In addition, it has also been reported that the administration of statins to hospitalized patients increases survival18 and decreases Candida burden in diabetic patients.19
Although the antifungal potential of statins has already been addressed in previous reports, studies involving the effect of statins on fungal biofilms are needed to obtain a better knowledge on the antifungal potential of these compounds. Hence, this study evaluated the effect of the statins simvastatin, atorvastatin, and pravastatin on planktonic cells of Candida spp. and Cryptococcus spp. Considering that the best results were obtained for simvastatin, this drug was evaluated in combination with antifungal drugs against planktonic growth. In addition, simvastatin was tested against biofilms of Candida spp. and Cryptococcus spp.
Materials and methodsMicroorganismsFor this study, 51 strains of Candida spp. (16 Candida albicans; 12 Candida tropicalis; 11 Candida krusei; 12 Candida parapsilosis sensu lato), and 25 strains of Cryptococcus spp. (13 C. neoformans – serotypes A, D and AD; and 12 Cryptococcus gattii – serotypes B and C) isolated from animals were used. The isolates belong to the culture collection of the Specialized Medical Mycology Center, Brazil. The purity and identity of the Candida spp. strains were confirmed by growth on chromogenic medium and microscopical and biochemical features.20 For the Cryptococcus spp. strains, capsule formation, melanin production, and biochemical testing were evaluated and the serotype of each strain was assessed by PCR.21
Susceptibility testing of planktonic cellsSusceptibility assays were performed using the broth microdilution method, as described by the document M27-A3 of the Clinical and Laboratory Standards Institute.22 The tests were performed in duplicate and visually read after 48h of incubation at 35°C. The strains C. parapsilosis ATCC 22019 and C. krusei ATCC 6258 were included as quality control for all tests.22
Inocula were prepared to obtain a final concentration of 0.5–2.5×103cellsmL−1.22 The statins simvastatin (Medley Indústria Farmacêutica Ltda, Campinas, SP, Brazil), atorvastatin (Laboratórios Pfizer Ltda, São Paulo, SP, Brazil), and pravastatin (Bristol-Myers-Squibb, Nova York, NY, USA) and the antifungal drugs amphotericin B (Sigma Chemical Corporation, St Louis, USA), itraconazole (Janssen Pharmaceutica, Beerse, Belgium), and fluconazole (Pfizer Pharmaceuticals, New York, USA) were tested against all strains.
To obtain the stock-solutions of each drug, atorvastatin, pravastatin, and fluconazole were diluted with sterile distilled water, and amphotericin B and itraconazole were diluted with dimethylsulfoxide (DMSO). Simvastatin was activated from its lactone prodrug form through hydrolysis in ethanolic NaOH (15% (v/v) ethanol, 0.25% (w/v) NaOH), at 60°C, for 1h.15 The concentration range tested was 3.9–2000μgmL−1 for simvastatin, 19.5–10,000μgmL−1 for atorvastatin, 97.6–50,000μgmL−1 for pravastatin, 0.0312–64μgmL−1 for amphotericin B and itraconazole, and 0.25–256μgmL−1 for fluconazole. The minimum inhibitory concentration (MIC) was defined as the lowest drug concentration able to inhibit 100% of fungal growth for amphotericin B and 50% inhibition of fungal growth when compared to the free-drug azoles22 and statins control.
As simvastatin provided the best antifungal results, we evaluated the interaction between this drug and the antifungal drugs against the tested yeasts. For drug interaction studies, simvastatin was tested with each azole by broth microdilution method, using the MIC of each tested drug alone as the highest concentrations tested in combination. The concentrations of the drugs in combination ranged from 0.03 to 1000, 0.00024 to 2, 0.00006 to 64 and 0.00048 to 256μgmL−1 for simvastatin, amphotericin B, itraconazole and fluconazole, respectively. The reading criteria were the same as for the antifungal drugs alone, namely 100% inhibition when combined with amphotericin B and 50% inhibition when combined with azoles. The interaction between the drugs was analyzed by calculating the fractional inhibitory concentration index (FICI), with values ≤0.5 indicating synergism.23
Susceptibility test of sessile cellsSimvastatin was tested against growing biofilms and mature biofilms of Candida spp. and Cryptococcus spp. Amphotericin B and itraconazole were used in all tests as control drugs for biofilm inhibition. The tests were performed in triplicate using one biofilm-producing strain of each tested fungal species (C. albicans, C. tropicalis, C. parapsilosis, C. krusei, C. neoformans and C. gattii), according to the methodology described by Chatzimoschou et al.,24 with some modifications. Briefly, strains were grown on Sabouraud dextrose agar for 48h at 30°C and then subcultured into Sabouraud dextrose broth for 24h, at 30°C, under agitation at 150rpm. After this period, the suspensions were centrifuged at 3000rpm for 10min, the supernatant was discarded, and the pellet was washed twice with sterile PBS. Then, the pellet was resuspended in RPMI 1640 medium (Gibco-BRL, USA), reaching a concentration of 1×106cellsmL−1. Tests were performed in 96-well polystyrene plates.
To evaluate the effect of simvastatin, amphotericin B, and itraconazole on growing biofilm, 100μL of the fungal suspension was exposed to 100μL of simvastatin and incubated at 35°C for 48h. The tested concentrations were based on the MIC obtained for each drug against fungal planktonic growth, including MIC, 10xMIC and 50xMIC. On the other hand, to evaluate the effect of simvastatin and the antifungals alone against mature biofilm, 100μL of the fungal suspension was added to 100μL of RPMI 1640 medium and incubated at 35°C for 48h. Then, the mature biofilms were exposed to simvastatin, amphotericin B, and itraconazole and incubated at 35°C for 48h. The tested concentrations against mature biofilms were 10xMIC, 50xMIC and 100xMIC. For all tests, drug-free growth control for each strain was included.
After 48h of drug exposure, the growing and mature biofilms were submitted to the following procedures: supernatants were collected and reserved for further analysis, and plates were washed twice with sterile PBS Tween 20 (0.05%, v/v) solution to remove non-adhered cells. Then, the biofilm viability was evaluated through XTT assay, according to Martinez and Casadevall,25 with modifications. Stock solutions of XTT (1mgmL−1) were previously prepared, filtrated and stocked at −20°C, until used. Menadione (Sigma) (0.4mM in acetone) was prepared at the moment of use. Afterwards, 50μL of sterile PBS, 75μL of XTT solution, and 6μL of menadione solution were added to each well. Plates were incubated at 35°C during 5h, in the dark, and then XTT was all transferred to a new plate and read in a spectrophotometer at 492nm.
Statistical analysisFor analysis of the MIC data of drugs against planktonic cells, Student's t test for independent and paired samples was used. To check the variation of the MIC values of the drugs in combination, as well as the FICI value, Student's t test for paired samples was also used. Regarding the biofilm assay, all tests were made in triplicate and results were evaluated by ANOVA and Tukey's multiple comparison post-test. p-Values<0.05 were considered statistically significant.
ResultsSusceptibility test of planktonic cellsAmong the tested statins, simvastatin showed the lowest MIC, with geometric means varying from 29.45 to 567.16 and from 62.5 to 500mgL−1 against the genera Candida and Cryptococcus, respectively (Table 1). Atorvastatin showed better results against Candida species, when compared to Cryptococcus spp., with MIC geometric means varying from 52.06 to 1682.37mgL−1 against Candida spp. and from 3886.02 to >10,000μgmL−1 against Cryptococcus spp. (Table 1). As for pravastatin, the MIC geometric means varied from 2159.24 to >50,000mgL−1 against Candida spp. and from 44079.56 to >50,000mgL−1 against Cryptococcus (Table 1). MIC geometric means for the classic antifungals against Candida spp. varied from 0.343 to 1.624mgL−1 for amphotericin B, from 0.046 to 15.021mgL−1 for itraconazole, and from 0.659 to 82.346mgL−1 for fluconazole. MIC geometric means for classic antifungals against Cryptococcus spp. varied from 0.125 to 0.735mgL−1 for amphotericin B, from 0.189 to 1mgL−1 for itraconazole, and from 6.498 to 64mgL−1 for fluconazole (Table 1).
Geometric means of the minimum inhibitory concentrations (MIC) of statins and antifungal agents against Candida spp. and Cryptococcus spp.
| Strains (n) | MIC geometric mean (μgmL−1) | |||||
|---|---|---|---|---|---|---|
| Statins | Antifungals | |||||
| Simvastatin | Atorvastatin | Pravastatin | Amphotericin B | Itraconazole | Fluconazole | |
| Candidaspecies | ||||||
| C. albicans (16) | 29.45 | 52.06 | 2159.24 | 0.561 | 5.992 | 21.357 |
| C. tropicalis (12) | 70.12 | 165.34 | 21022.41 | 0.343 | 15.021 | 82.346 |
| C. krusei (11) | 567.16 | 755.06 | >50,000 | 1.624 | 0.072 | 12.126 |
| C. parapsilosis sensu lato (12) | 235.97 | 1491.37 | >50,000 | 0.707 | 0.059 | 0.891 |
| Cryptococcusspecies | ||||||
| C. neoformans Aa (11) | 500 | 3886.02 | 44079.56 | 0.536 | 0.189 | 6.498 |
| C. neoformans D (1) | 250 | >10,000 | >50,000 | 0.25 | 0.25 | 8 |
| C. neoformans AD (1) | 62.5 | >10,000 | >50,000 | 0.125 | 0.5 | 8 |
| C. gatti B (11) | 500 | 5325.21 | 44079.56 | 0.735 | 0.315 | 34.562 |
| C. gatti C (1) | 500 | >10,000 | >50,000 | 1 | 1 | 64 |
Results for the in vitro interaction between simvastatin and antifungal drugs against these yeasts are shown in Table 2. In general, a synergistic interaction was observed between simvastatin and both azoles against C. albicans (n=10/10), C. tropicalis (n=11/11) and C. parapsilosis sensu lato (n=12/12) (p<0.05). Concerning C. krusei, only the combination of simvastatin and itraconazole was synergistic (n=9/10) (p<0.05). As for Cryptococcus spp., synergistic interactions were observed between simvastatin and the three antifungals tested against C. gattii (simvastatin/amphotericin B and simvastatin/fluconazole: n=7/7; simvastatin/itraconazole: n=5/7) and C. neoformans (simvastatin/amphotericin B: n=11/11; simvastatin/itraconazole: n=9/11; simvastatin/fluconazole: n=10/11) (p<0.05).
Geometric means of the minimum inhibitory concentrations (MIC) of the combination of simvastatin and the antifungal amphotericin B, itraconazole, or fluconazole against Candida spp. and Cryptococcus spp.
| Species (n) | Drugs | MIC (isolated) (μgmL−1) | MIC (combination) (μgmL−1) | FICI | Number of strains showing synergism | ||
|---|---|---|---|---|---|---|---|
| SIM | Antifungal | SIM | Antifungal | ||||
| Candida albicans (10) | |||||||
| SIM/AMB | 29.11 | 0.616 | 24.06 | 0.595 | 1.741 | 0/10 | |
| SIM/ITC | 29.11 | 9.189 | 1.042 | 0.329 | 0.071 | 10/10 | |
| SIM/FLC | 29.11 | 21.112 | 1.695 | 0.116 | 0.116 | 10/10 | |
| Candida tropicalis (11) | |||||||
| SIM/AMB | 75.47 | 0.3426 | 55.637 | 0.354 | 1.37 | 1/11 | |
| SIM/ITC | 75.47 | 15.021 | 1.327 | 0.266 | 0.035 | 11/11 | |
| SIM/FLC | 75.47 | 82.347 | 4.425 | 4.832 | 0.117 | 11/11 | |
| Candida krusei (10) | |||||||
| SIM/AMB | 574.35 | 1.624 | 435.28 | 1.231 | 1.515 | 0/10 | |
| SIM/ITC | 574.35 | 0.072 | 116.61 | 0.014 | 0.416 | 9/10 | |
| SIM/FLC | 574.35 | 12.126 | 233.26 | 4.925 | 0.812 | 3/10 | |
| Candida parapsilosis sensu lato (12) | |||||||
| SIM/AMB | 235.97 | 0.707 | 78.74 | 0.236 | 0.667 | 7/12 | |
| SIM/ITC | 235.97 | 0.059 | 41.68 | 0.010 | 0.353 | 12/12 | |
| SIM/FLC | 235.97 | 0.891 | 49.58 | 0.187 | 0.420 | 12/12 | |
| Cryptococcus neoformans (11) | |||||||
| Serotypes: A(9); D(1); AD(1) | SIM/AMB | 388.6 | 0.4139 | 35.42 | 0.0377 | 0.182 | 11/11 |
| SIM/ITC | 388.6 | 0.1943 | 70.86 | 0.0354 | 0.365 | 9/11 | |
| SIM/FLC | 388.6 | 6.622 | 21.38 | 0.3648 | 0.11 | 10/11 | |
| Cryptococcus gattii (7) | |||||||
| Serotypes: B(6); C(1) | SIM/AMB | 500 | 0.8203 | 19.025 | 0.0312 | 0.076 | 7/7 |
| SIM/ITC | 500 | 0.4102 | 84.0803 | 0.0689 | 0.336 | 5/7 | |
| SIM/FLC | 500 | 39.008 | 38.051 | 2.972 | 0.152 | 7/7 | |
SIM, simvastatin; AMB, amphotericin B; ITC, itraconazole; FLC, fluconazole; MIC, minimum inhibitory concentration; FICI, fractional inhibitory concentration index.
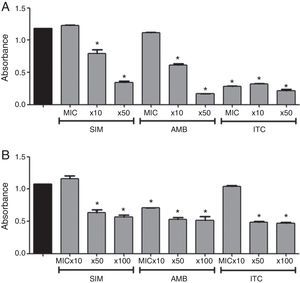
Regarding the action of simvastatin against biofilm of Candida spp., simvastatin inhibited growing biofilms at concentrations greater than 10xMIC (Fig. 1). Amphotericin B caused significant decrease in metabolic activity of growing biofilms at 10xMIC and 50xMIC, while itraconazole caused inhibition at all tested concentrations (p<0.05). As for mature biofilms, simvastatin caused significant decrease in metabolic activity (Fig. 1) (p<0.05), at concentrations above 50xMIC. Amphotericin B inhibited mature biofilms at all tested concentrations, while itraconazole only at 50xMIC and 100xMIC (p<0.05).
In vitro effect of simvastatin (SIM), amphotericin B (AMB), and itraconazole (ITC) at 3 different concentrations on biofilm formation (A) and mature biofilm (B) of Candida spp. Black bars: positive control; MIC: minimum inhibitory concentration. Absorbance of XTT (492nm). *Represents statistically significant difference (p<0.05) when compared to the positive control. Data expressed as mean±SEM.
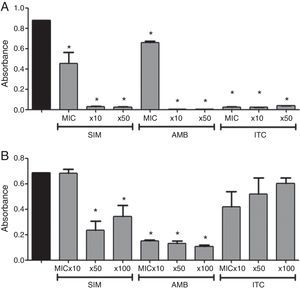
Regarding the genus Cryptococcus, simvastatin inhibited growing biofilms at all tested concentrations (Fig. 2) (p<0.05), similar to what was observed when amphotericin B and itraconazole were used (p<0.05). Concerning mature biofilms, simvastatin caused significant decrease in metabolic activity of Cryptococcus biofilm at 50xMIC and 100xMIC (Fig. 2) (p<0.05). Amphotericin B inhibited mature biofilms at all tested concentrations (p<0.05), while itraconazole did not decrease biofilm metabolic activity at any tested concentration.
In vitro effect of simvastatin (SIM), amphotericin B (AMB), and itraconazole (ITC) at 3 different concentrations on biofilm formation (A) and mature biofilm (B) of Cryptococcus spp. strains. Black bars: positive control; MIC, minimum inhibitory concentration. Absorbance of XTT (492nm). *Represents statistical significant difference (p<0.05) when compared to the positive control. Data expressed as mean±SEM.
This study shows the inhibitory activity of simvastatin on the growth of yeasts of the genera Candida and Cryptococcus with an inhibitory effect against both planktonic cells and biofilms. The MICs of simvastatin against C. albicans and C. tropicalis were similar to the serum levels of the drug, when administered to control blood cholesterol.26C. albicans and C. tropicalis are important fungal pathogens commonly isolated from candidemia.27,28 Simvastatin and atorvastatin have been described inhibiting Candida spp. and the filamentous fungus Aspergillus fumigatus.13 This work confirms the action of these two drugs, especially simvastatin against Candida spp. and Cryptococcus spp. However, the growth of these fungi was not inhibited by pravastatin. The use of statins deregulates cellular production of isoprenoid,13 which leads to mitochondrial dysfunction, respiratory deficit,29 and changes in lipid structure and in the dynamics of plasma membrane of C. albicans cells.30
There is no synergistic interaction when simvastatin is associated with amphotericin B against most Candida spp. strains. Statins reduce the amount of fungal ergosterol, which may lead to decreased activity of amphotericin B, since ergosterol is the target molecule for this antifungal drug and a decrease in the amount of this molecule is one of the mechanisms developed by amphotericin B resistant Candida spp. However, synergism between simvastatin and amphotericin B was observed against strains of Cryptococcus spp., in line with previous reports with the filamentous fungi Rhizopus oryzae and Aspergillus flavus.31 These contradictory findings still need to be elucidated.
In general, when simvastatin is associated with azoles (i.e. itraconazole or fluconazole) there is synergism against strains of Candida spp. and Cryptococcus spp. However, probably due to the intrinsic resistance of C. krusei to fluconazole, synergism between simvastatin and fluconazole was not observed against this Candida species. The interaction between statins and azoles has been reported against the yeasts Saccharomyces cerevisiae32 and Candida spp.,33 and the filamentous fungi Aspergillus spp., Mucor spp. and Rhizopus spp.17,33 Synergism between these two pharmacological groups is most likely associated with the combined action of the drugs in reducing fungal ergosterol, by acting at different moments in the pathway of ergosterol biosynthesis.11 In addition, the reduction of endogenous sterol due to the action of statins increases cell membrane permeability in order to increase absorption of exogenous sterol, as a compensatory mechanism, and, simultaneously, the entrance of azoles in the cell is facilitated.34
Biofilm production is considered an important virulence factor of Candida spp.35 and it contributes for the persistence of infections.36 It has been demonstrated that simvastatin also inhibits growing and mature biofilms of Candida spp. and Cryptococcus spp. strains when used alone. Liu et al.37 showed that simvastatin inhibited biofilm production of C. albicans after 16-h-incubation, suggesting that at least one mechanism of inhibition involves interference with ergosterol biosynthesis. On the other hand, there are no reports of the effect of simvastatin on biofilms of Cryptococcus spp. Additional studies are needed to better understand the action of simvastatin against yeast biofilms.
Studies have shown that amphotericin B inhibits fungal biofilms25,38 causing apoptosis of the cells in Candida biofilms.39 In our study, growing and mature biofilms of Candida spp. and Cryptococcus spp. were inhibited by amphotericin B. Although many authors have reported that azoles do not inhibit fungal biofilms,25,38 the present study demonstrated the inhibition of growing and mature biofilms of Candida spp. by itraconazole. As for Cryptococcus spp., itraconazole only inhibited growing biofilms.
Although several reports have demonstrated in vitro activity of statins agents against many clinical relevant yeast and mold species, as well as the synergistic effect of statins with different antifungal drugs, clinical studies are scarce.40 Spanakis et al.19 showed that use of statins decreased the incidence of cultures positive for Candida species among patients with type 2 diabetes mellitus who underwent gastrointestinal surgery. In contrast, no beneficial effects were observed for statins in a study of patients with candidemia.41 However, these studies were performed with different patient groups and were inconclusive. Thus, further studies aiming to evaluate the benefits of statin in antifungal therapy are required.
The present study showed the activity of statins against Candida and Cryptococcus species, with particular emphasis on simvastatin, isolated and combined with classical antifungals. In addition, it was also demonstrated that simvastatin was able to inhibit growing and mature biofilms of Candida spp. and Cryptococcus spp.
Conflicts of interestThe authors declare no conflicts of interest.
This work was supported by grants from the National Council for Scientific and Technological Development (CNPq; Brazil; Processes 307606/2013-9; 443167/2014-1; 445670/2014-2) and CAPES (AE1 – 0052-000650100/11).