Streptococcus pneumoniae, which cause noninvasive pneumococcal diseases, severely impair children's health. This study analyzed serotype distribution and antimicrobial resistance of S. pneumoniae from January 2012 to December 2012 in a Children's Hospital, Shanghai.
MethodsA total of 328 pneumococcal isolates were serotyped by multiplex sequential PCR and/or capsule-quellung reaction. The minimum inhibitory concentrations for 11 antimicrobial agents were determined by broth microdilution method.
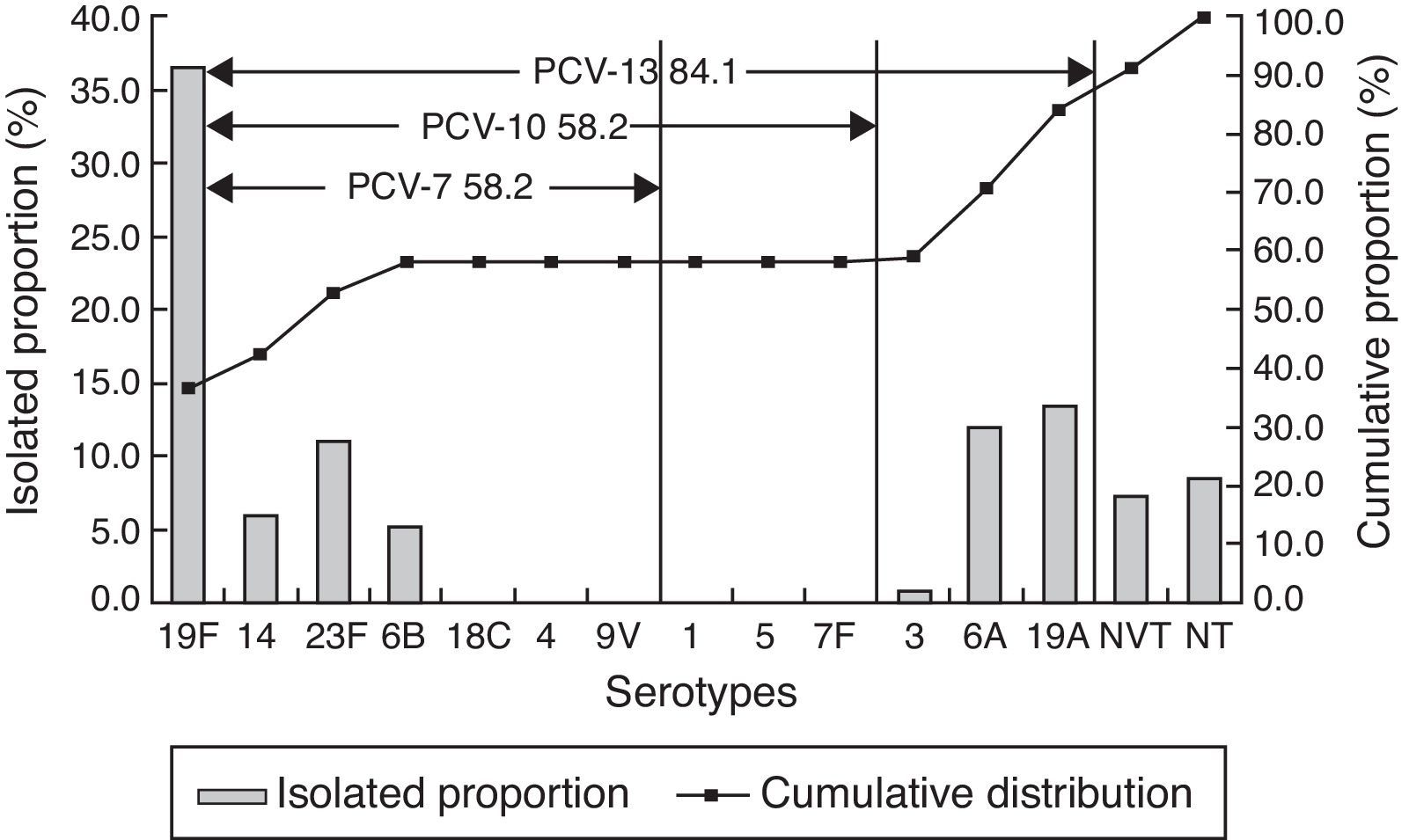
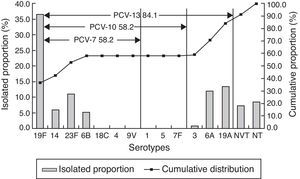
ResultsAmong 328 strains, 19F (36.3%), 19A (13.4%), 6A (11.9%), 23F (11.0%), 14 (5.8%), 6B (5.2%), and 15B/C (4.3%) were the most common serotypes. The coverage rates of 7-, 10-, and 13-valent conjugate vaccines (PCV7, PCV10, and PCV13) were 58.2%, 58.2%, and 84.1%, respectively. Out of the isolates, 26 (7.9%) strains were penicillin resistant. Most of the strains displayed high resistance rate to macrolides (98.5% to erythromycin, 97.9% to azithromycin, and 97.0% to clindamycin).
ConclusionsThe potential coverage of PCV13 is higher than PCV7 and PCV10 because of the emergence of 19A and there should be long-term and systematic surveillance for non-vaccine serotypes.
Streptococcus pneumoniae remains a major pathogen of pneumonia, meningitis and acute otitis in children, with high morbidity and mortality. According to a survey conducted by the World Health Organization (WHO), 50–60% of the 1.6 million deaths caused by pneumococcal infections in 2005 were in children younger than five years old, especially in developing countries.1
Heptavalent pneumococcal conjugate vaccine (PCV7), targeting seven of more than 90 serotypes of S. pneumoniae (serotypes 4, 6B, 9V, 14, 18C, 19F, 23F), has reduced the burden of invasive pneumococcal diseases (IPD) and nasopharyngeal carriage rate of these serotypes in vaccinated children in developed countries.2,3 Moreover, several reports indicate that in some countries PCV7 is also effective against noninvasive pneumococcal disease (NIPD) like pneumonia.4,5 In USA, Grijalva et al.4 reported that the all-cause pneumonia admission rates had declined by 39% for children younger than two years following licensure of PCV7. However, after the introduction of PCV7 the incidence of pneumococcal diseases caused by non-PCV7 serotypes such as 6A and 19A increased.6,7 The Food and Drug Administration (FDA) approved two more vaccines: the 10-valent pneumococcal conjugate vaccine (PCV10) including serotypes 1, 5, and 7F in addition to PCV7 serotypes and the 13-valent pneumococcal conjugate vaccine (PCV13) including serotypes 3, 6A and 19A in addition to PCV10 serotypes. Both proved to be more effective than PCV7.8–9
In Mainland China, PCV7 has not yet been introduced into childhood immunization program despite being available in the market since 2008. Information about the use of PCV10 and PCV13 in Mainland China has also been unclear. It has been proposed that the choice of S. pneumoniae vaccine should be based on the distribution of serotypes, which vary with age, geographic region and time.10 Therefore, systematic and accurate assessment of the serotype distribution of S. pneumoniae is necessary for choosing an optimal vaccine.
Interestingly, NIPD accounts for a major proportion of all pneumococcal infections. O’Brien et al.11 reported that out of 14.5 million pneumococcal cases, 95.6% were cases of NIPD while only 4.4% were IPD. However, data about S. pneumoniae isolated from children with NIPD is rare. Moreover, continued surveillance of the distribution of pneumococcal serogroup isolated from specimens of non-sterile sites may help determining which serogroups are important in the development of invasive disease.5 In this study, we investigated S. pneumoniae isolated from children with NIPD in 2012. The purpose of this surveillance study was to determine the serotype distribution and antimicrobial resistance pattern of S. pneumoniae strains causing NIPD in Shanghai, a metropolitan city located in the east of China. Such information could provide guidance for further clinical and epidemiologic studies, rational administration of antimicrobial agents, and selection of proper vaccines.
Material and methodsStudy design and data collectionThis surveillance was conducted between January 2012 and December 2012 in Shanghai Children's Hospital, which is one of the largest pediatric hospitals in Shanghai. The hospital serves 1.13 million outpatients and 20,000 inpatient admissions annually. All specimens were collected from children (0–14 years of age) who were hospitalized and diagnosed with NIPD. NIPD was defined as S. pneumoniae strains causing infection detected in ear, eye, nasopharynx, or tracheal aspirate specimens and in which no invasive (sterile sites) isolates were collected from the same patient.12 Furthermore, the clinical data of enrolled patients including demographics, admission and/or discharge diagnosis, time of sampling, recent use of antimicrobial agents, vaccination history were obtained from the medical records. If two isolates expressing the same serotype from the same patient, only one isolate was included with duplicates being discarded.
Clinical isolates and microbiological testsAll specimens were processed in a microbiology laboratory according to the “National Guide to Clinical Laboratory Procedures”.13 A total of 328 non-duplicated strains were included. The samples were sent to the microbiology laboratory and inoculated on 5% sheep blood agar plates. The plates were incubated at 37°C in a 5% CO2 incubator and examined after 24–48h. Typical colonies of S. pneumoniae were subcultured and identified by using the optochin disk (Oxoid, Hampshire, UK) and bile solubility test.14 All strains were stored at −80°C in 40% glycerol broth medium until further analysis. Pneumococcal isolates were serotyped by multiplex sequential PCR15 and/or capsule-quellung reaction16 with a set of antisera from the Statens Serum Institute (Copenhagen, Denmark). The minimum inhibitory concentrations (MICs) for penicillin (PEN), cefuroxime (CXM), ceftriaxone (CRO), erythromycin (ERY), azithromycin (AZM), clindamycin (CLI), levofloxacin (LEV), moxifloxacin (MXF), vancomycin (VAN) and trimethoprim-sulfamethoxazole (SXT) were determined by broth microdilution method according to the 2013 guidelines of the Clinical and Laboratory Standards Institute (CLSI M100-S23). S. pneumoniae ATCC 49619 was used as the control strain for the susceptibility test.
Statistical analysisWhonet 5.6 software17 and SPSS 16.0 (Statistical Package for Social Science, USA) were used for susceptibility statistics and analysis. The χ2 test or Fisher's exact test of program were used for comparing categorical data. Two-tailed p-values <0.05 were considered statistically significant.
ResultsDemographic data of patientsA total of 328 isolates of S. pneumoniae were included being 61.3% (201/328) detected in male patients and 38.7% (127/328) in females. Patients aged between 0 and 14 years old were divided into four groups: infant (<1 year, 37.8%), toddler (1–2 years, 28.4%), preschooler (3–5 years, 27.7%) and schooler (6–14 years, 6.1%). Upper respiratory tract infections were diagnosed in 243 children (74.1%) and 35 (10.7%) had a history of contact with other infected patients. All of these patients had history of prior hospitalization or recent physician visit and therapy with antibiotics. Only four children (1.2%) had received PCV-7 vaccination.
Serotype distributionOut of 328 pneumococcal isolates, 300 strains (91.5%) were successfully typed with 14 serotypes by multiplex PCR and capsule-quellung reaction. Serotype 19F was the predominant serotype (36.3%), followed by 19A (13.4%), 6A (11.9%), 23F (11.0%), 14 (5.8%), 6B (5.2%), 15B/C (4.3%). Another eight serotypes were detected in fewer than five strains, which included serotype 15A (4), 3 (2), 35B (2), 10A (1), 11A (1), 7C (1), 17F (1). The coverage rates of PCV7, PCV10, and PCV13 serotypes were 58.2%, 58.2% and 84.1%, respectively (Fig. 1).
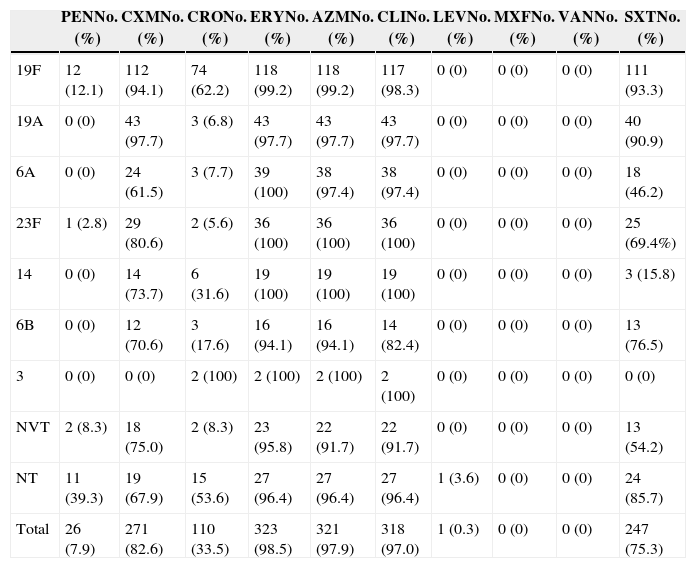
Antimicrobial susceptibilityAccording to CLSI 2013 breakpoints for parenteral penicillin (resistant ≥8μg/mL and intermediate 4μg/mL for non-meningitis isolates), the prevalence rate of penicillin non-susceptible S. pneumoniae (PNSP) was 28.6%, including 20.7% penicillin intermediate S. pneumoniae (PISP) and 7.9% penicillin resistance S. pneumoniae (PRSP). Resistance to cefuroxime was seen in 82.6% of isolates whereas 33.5% was resistant to ceftriaxone. Most of the strains displayed high resistance rate to macrolides (98.5% to erythromycin, 97.9% to azithromycin) and to clindamycin (97.0%).
In sharp contrast, all of these isolates were susceptible to levofloxacin, moxifloxacin and vancomycin with the exception of one strain (0.3%) that was resistant to levofloxacin and intermediate to moxifloxacin. In addition, the resistance rate to trimethoprim-sulfamethoxazole was 75.3%.
Association between antimicrobial susceptibility and serotypeWe further assessed antimicrobial susceptibility of S. pneumoniae among different serotypes (Table 1). Serotype 19F showed the highest resistance rate to penicillin, ceftriaxone and trimethoprim-sulfamethoxazole. The highest prevalence of cefuroxime resistance was observed with serotype 19A. All of 14 serotypes exhibited high resistance rates to macrolides and clindamycin.
Antimicrobial resistance rates of 328 S. pneumoniae isolates of different serotypes against 10 antimicrobial agents.
| PENNo. (%) | CXMNo. (%) | CRONo. (%) | ERYNo. (%) | AZMNo. (%) | CLINo. (%) | LEVNo. (%) | MXFNo. (%) | VANNo. (%) | SXTNo. (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| 19F | 12 (12.1) | 112 (94.1) | 74 (62.2) | 118 (99.2) | 118 (99.2) | 117 (98.3) | 0 (0) | 0 (0) | 0 (0) | 111 (93.3) |
| 19A | 0 (0) | 43 (97.7) | 3 (6.8) | 43 (97.7) | 43 (97.7) | 43 (97.7) | 0 (0) | 0 (0) | 0 (0) | 40 (90.9) |
| 6A | 0 (0) | 24 (61.5) | 3 (7.7) | 39 (100) | 38 (97.4) | 38 (97.4) | 0 (0) | 0 (0) | 0 (0) | 18 (46.2) |
| 23F | 1 (2.8) | 29 (80.6) | 2 (5.6) | 36 (100) | 36 (100) | 36 (100) | 0 (0) | 0 (0) | 0 (0) | 25 (69.4%) |
| 14 | 0 (0) | 14 (73.7) | 6 (31.6) | 19 (100) | 19 (100) | 19 (100) | 0 (0) | 0 (0) | 0 (0) | 3 (15.8) |
| 6B | 0 (0) | 12 (70.6) | 3 (17.6) | 16 (94.1) | 16 (94.1) | 14 (82.4) | 0 (0) | 0 (0) | 0 (0) | 13 (76.5) |
| 3 | 0 (0) | 0 (0) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| NVT | 2 (8.3) | 18 (75.0) | 2 (8.3) | 23 (95.8) | 22 (91.7) | 22 (91.7) | 0 (0) | 0 (0) | 0 (0) | 13 (54.2) |
| NT | 11 (39.3) | 19 (67.9) | 15 (53.6) | 27 (96.4) | 27 (96.4) | 27 (96.4) | 1 (3.6) | 0 (0) | 0 (0) | 24 (85.7) |
| Total | 26 (7.9) | 271 (82.6) | 110 (33.5) | 323 (98.5) | 321 (97.9) | 318 (97.0) | 1 (0.3) | 0 (0) | 0 (0) | 247 (75.3) |
PEN, penicillin; CXM, cefuroxime; CRO, ceftriaxone; ERY, erythromycin; AZM, azithromycin; CLI, clindamycin; LEV, levofloxacin; MXF, moxifloxacin; VAN, vancomycin; SXT; trimethoprim-sulfamethoxazole.
Furthermore, among antimicrobial non-susceptible isolates including β-lactamase, macrolides and sulfonamides, the coverage rates of PCV7 types were significantly higher than non-PCV7 types (p<0.05). The common serotypes in PNSP were 19F and 19A, accounting for 68.1% and 10.6%, respectively.
DiscussionsPneumococcal conjugate vaccine, development of which is based on the local serotype distribution, is one of the important measures to prevent invasive and noninvasive infections by S. pneumoniae and also prevent the spread of drug resistance.1 Owing to capsular polysaccharide, S. pneumoniae can be differentiated into 90 distinct serotypes but only a few can cause serious diseases.18
PCV7, firstly introduced in 2000 in USA, was recommended as a part of routine childhood immunization by the WHO in 2007. However, after wide application of PCV7, “serotype replacement”, a phenomenon that PCV7 serotypes decreased with concurrent increase of NVTs among invasive and noninvasive diseases was observed.6 Similarly, this study demonstrated that 19F, 19A, 6A and 23F were the most frequent serotypes, as had been observed in another study conducted by Yao et al. in Mainland China.19 Out of the most common serotypes, 19A and 6A are non-PCV7 serotypes and represented 25.3% of all isolates. As reported by Hackel et al.,20 19A became the most common serotype in children less than five years after introduction of PCV7 throughout the world. In the United States, the prevailing serotypes causing NIPD between 1999–2011 were 19A, 3 and 35B, which increased from 2% to 22%, 8.5% to 9.3%, 4.0% to 7.0%, respectively.21 A review shows that in Europe the most common isolates were serotypes 1, 19A, 3, 6A, and 7F after PCV7 introduction in Belgium, France, Germany, Greece, Norway, Portugal, Spain, and the UK.22 Therefore, there should be long-term and systematic surveillance for non-vaccine serotypes, especially 19A serotype.
In addition, the coverage rates of PCV7 also changed under the same condition. In Europe, vaccine serotype coverage rate ranged from 37% to 100% for PCV7, with mean increases in coverage of 7% and 16% for PCV10 and PCV13, respectively.22 Furthermore, in Hong Kong, the proportion of PCV7 serotypes on IPD declined from 89.5% to 65.7% but that of PCV13 remained stable (91.4–93.2%).23 A Chinese multicenter study on NIPD of children19 showed that PCV7, PCV10, and PCV13 covered 76.3%, 76.9%, and 92.3% of isolates, respectively. However, our study suggested that the coverage rates of PCV7, PCV10, and PCV13 were 58.2%, 58.2%, and 84.1%, respectively, lower than other studies,24,25 which may be the result of increasing non-PCV types in Mainland China.
Antimicrobial agents, especially penicillin, are always used for treating pneumococcal infections. However, since the PRSP isolates were first discovered in Australia, the phenomenon of S. pneumoniae antimicrobial resistance has posed a major challenge. In the United States, resistance rates for penicillin increased from 5.3% to 15.9% from 2004 to 2009.26 The proportions of PNSP throughout Europe was 0.4% in Belgium, 3.1% in UK, 3.7% in Germany, 9.2% in Italy, 15% in Hungary, and 27.6% in France.27 Among Asian countries, the PNSP rate was 13.2% in China, 1.5% in Hong Kong, and 2.2% in South Korea.28 Despite the decline of non-susceptibility rate with the revision of the new CLSI breakpoints in developed countries, the rate of PNSP in China remained high.19,29
At present, it is remarkable the relationship between penicillin resistance and serotypes. In Japan, significantly higher rates of penicillin resistant isolates were mostly found in 19F and 23F serotypes.30 Half of the penicillin resistant isolates were identified as serotype 19F in China.19 However, some surveys in western countries have reported that non-PCV7 serotypes, 19A in particular, have become not only more prevalent, but also more resistant to antimicrobials. In the United States21 most PRSP isolates were serotypes 19A (75.3%), 35B (11.1%), or 19F (7.2%). Moreover, we detected that 19A was also the second most frequent serotype and made up for 10.6% of PNSP. All of these findings suggest that PCV7 vaccination may not be the sole factor for the observed increase of serotype 19A in areas where PCV7 was widely used, and that antimicrobial abuse may also play a vital role in the serotype replacement phenomenon.19
In conclusion, the current study examined the serotype distribution and antimicrobial resistance of noninvasive pneumococcal infections in Shanghai, China. Our data demonstrated that the use of PCV7, PCV10 and PCV13 has the potential to decrease the incidence of the pneumococcal infections, similarly to what has been observed in developed countries. Hence, long-term surveillance of S. pneumoniae is required to monitor the dynamic changing of serotypes and antimicrobial resistance, which would likely provide the basis for choosing optimal vaccines for the prevention of pneumococcal infections and developing new and more effective vaccines.
This study has several limitations. Firstly, all of the NIPD strains were isolated from pediatric patients from one hospital in China. Additionally, this study only analyzed the NIPD specimens, without inclusion of isolates from invasive pneumococcal diseases. Therefore, a multicenter study including isolates from more pediatric hospitals should be conducted.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank all the patients who contributed their specimens and clinical data for this study. We thank the microbiologists and technical staff of Shanghai Children's Hospital for collecting the bacterial isolates and laboratory testing. This study was partly supported by the Shanghai Municipal Commission of Health and Family Planning (funding # 20124026).