Homeless persons have been considered as one of the most susceptible populations to sexually transmitted infections. In Brazil, these population experienced an increase of 140% from 2012 to 2020. Accordingly, the present study aimed to assess the seroprevalence of anti-Treponema pallidum, anti-HCV, anti-HIV antibodies, and the risk factors associated with homeless persons in a daytime attendance shelter of São Paulo city during the syphilis epidemic in Brazil. Blood samples of 116 volunteers and epidemiological data were conveniently collected in the shelter from June through August 2018. Detection of syphilis, HCV, and HIV antibodies was performed by chemiluminescent microparticle immunoassay (CMIA). CMIA-reagent samples for anti-T. pallidum antibodies were confirmed by Venereal Disease Research Laboratory (VDRL) non-treponemal test. VDRL non-reagent samples were confirmed by treponemal rapid immunochromatographic test. A rapid immunoblot assay confirmed seropositivity to HIV. Overall, anti-T. pallidum antibodies were observed in 29/116 (25.0%), anti-HCV antibodies in 4/116 (3.4%), and anti-HIV antibodies in 2/116 (1.7%) individuals, both co-infected with anti-T. pallidum antibodies. Associated risk factors for syphilis in homeless persons were being born or previously living in another city (p = 0.043) and becoming homeless due to family conflicts (p = 0.035). Besides homeless vulnerability, worldwide shortage of benzathine penicillin supply and increasing of syphilis testing access through rapid testing in primary health care services may have also impacted disease spreading at the time. The prevalence of syphilis found herein is the highest worldwide to date in this population.
Homeless persons have been considered one of the most susceptible populations to sexually transmitted infections (STI) such as those caused by Treponema pallidum, hepatitis C virus (HCV), and human immunodeficiency virus (HIV), mostly due to social vulnerability and limited access to preventive care and health services.1,2
Similarly, other infectious diseases frequently occur in this population.3,4 A recent report estimates 221,869 homeless persons living in Brazil, of which 24,344 in São Paulo city. There was a 140% increase in the country's homeless population from 2012 to 2020, mostly associated with the economic crisis leading to unemployment and poverty.5,6
Homeless persons are at increased risk of acquiring infectious diseases due to the limited access to treatment and prevention programs, poor hygiene, increased use of alcohol and injectable drugs, and unprotected sex.7,8 The present study aimed to investigate the presence of antibodies against syphilis, HCV, and HIV in homeless persons in São Paulo, Brazil, the largest city in Latin America.
Material and methodsThis study was a descriptive cross-sectional seroepidemiological approach of a homeless population in a major daytime attendance shelter of western São Paulo city. The research was conducted along with the city's multi-professional team of the Secretary of Health, called "Street Outreach Office", part of the Brazilian Unified Health System (SUS).9 This study was approved by the Ethics Committee in Human Research of the Federal University of Paraná (CAAE: 80099017.3.0000.0102, protocol number: 2.512.196), by the Ethics Committee in Human Health of the São Paulo City Secretary of Health (CAAE: 80099017.3.3004.0086, protocol number: 3.366.684) and by the Ethics Committee in Human Research of the Clinics Hospital at the Federal University of Paraná (CAAE: 80099017.3.3005.0096, protocol number: 3.623.845). The patients/participants provided their written informed consent to participate in this study.
Homeless persons were recruited by city health officials and invited to voluntarily participate in the research. Blood samples of 116 volunteers were conveniently collected from June to August 2018, which was the permitted timeframe. Epidemiological data collection was carried out using a questionnaire designed for homeless persons; refusal to fully or partially answer any question or incomplete answers was accepted and registered.
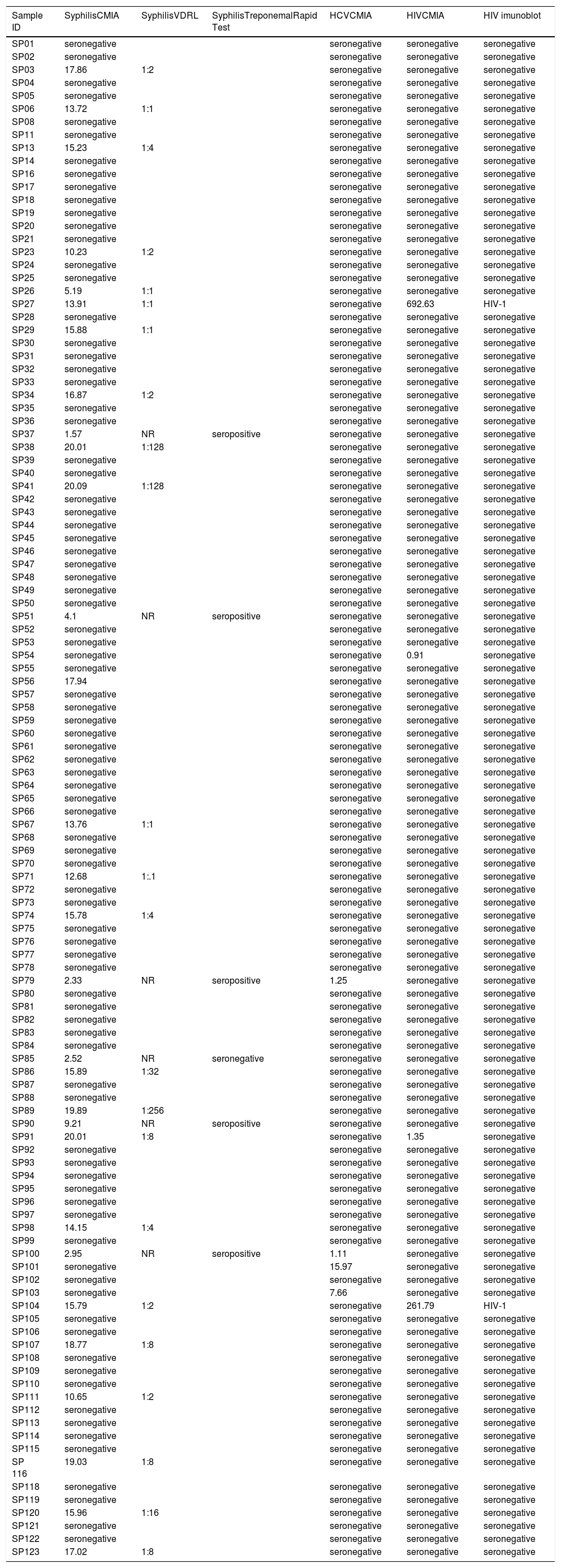
Detection of syphilis, HCV and HIV was performed by chemiluminescent microparticle immunoassay (CMIA) (Anility i Syphilis TP, Anti-HCV, HIVAg/Ab, Abbott Laboratories, Chicago, IL, USA). Cases of reactive serology for HIV were confirmed by a rapid immunoblot test (DPP HIV1/2®, Fiocruz, Rio de Janeiro, Brazil), as recommendations of the Brazilian Ministry of Health.10 Although screening for viral hepatitis B by rapid test has been also incorporated by SUS, the São Paulo Secretary of Health had a shortage of these tests throughout 2018. CMIA-reagent samples for anti-T. pallidum antibodies were confirmed by Venereal Disease Research Laboratory (VDRL) non-treponemal test. VDRL non-reagent samples were confirmed by treponemal rapid immunochromatographic test (MedTeste Sífilis MedLevensohn®, São Paulo, Brazil), as recommended by SUS.11 Reagent results may represent early syphilis in recent infection or untreated late syphilis, also in activity.11
Statistical analysis was performed using SPSS 20.0.12 Frequencies of syphilis and HCV seropositivity (absolute and relative) were determined by the stratification of the observations according to variables. Chi-Square test was used to determine univariate association between studied variables, and odds ratios (OR) were used to assess the association between syphilis and HCV prevalence and potential risk factors. Association between factors was considered when p < 0.05.
ResultsIn short, profile of surveyed homeless volunteers included 103/116 (88.8%) males, 105/116 (90.5%) unmarried and 89/116 (76.7%) non-white individuals. A total of 77/116 (66.4%) persons had none to 8th grade as educational background and 97/116 (83.6%) declared receiving no income at the time. While 29/116 (25.0%) individuals were assisted by psychosocial service, 87/116 (75.0%) persons referred use of legal or illegal substances, mostly alcohol in 62/87 (71.3%), followed by tobacco in 32/87 (36.8%) and cocaine in 31/87 (35.6%) individuals. São Paulo was not the city of birth for 80/116 (69.0%) individuals, 64/116 (55.2%) slept in shelters at night, and family conflicts was the main reported reason to have become homeless in 47/116 (40.5%) answers (Table 1).
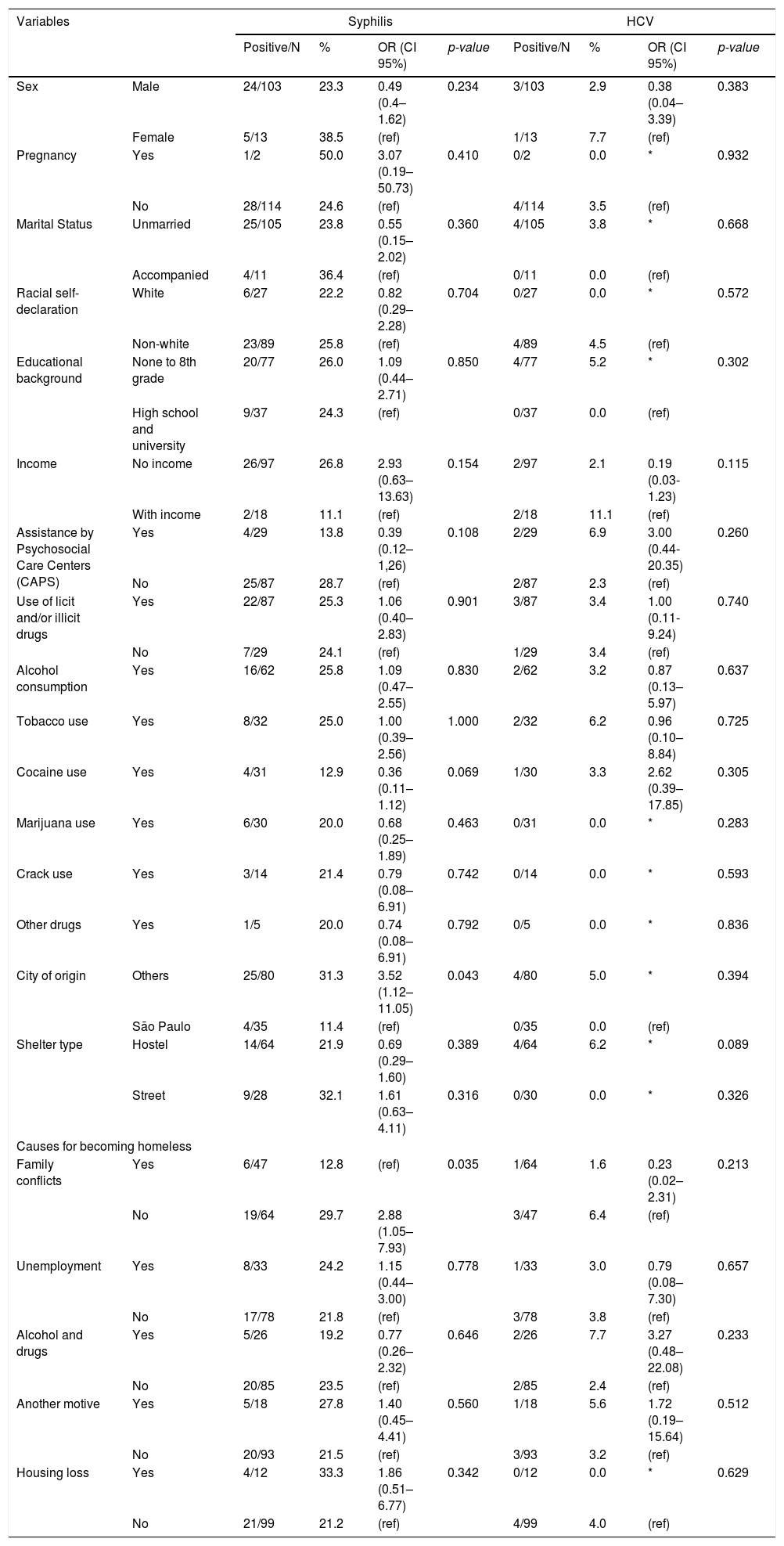
Statistical results of univariate and multiple logistic regression models of associated risk factors for seropositivity of anti- Treponema pallidum and anti- HCV antibodies in homeless persons.
| Variables | Syphilis | HCV | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Positive/N | % | OR (CI 95%) | p-value | Positive/N | % | OR (CI 95%) | p-value | ||
| Sex | Male | 24/103 | 23.3 | 0.49 (0.4–1.62) | 0.234 | 3/103 | 2.9 | 0.38 (0.04–3.39) | 0.383 |
| Female | 5/13 | 38.5 | (ref) | 1/13 | 7.7 | (ref) | |||
| Pregnancy | Yes | 1/2 | 50.0 | 3.07 (0.19–50.73) | 0.410 | 0/2 | 0.0 | * | 0.932 |
| No | 28/114 | 24.6 | (ref) | 4/114 | 3.5 | (ref) | |||
| Marital Status | Unmarried | 25/105 | 23.8 | 0.55 (0.15–2.02) | 0.360 | 4/105 | 3.8 | * | 0.668 |
| Accompanied | 4/11 | 36.4 | (ref) | 0/11 | 0.0 | (ref) | |||
| Racial self-declaration | White | 6/27 | 22.2 | 0.82 (0.29–2.28) | 0.704 | 0/27 | 0.0 | * | 0.572 |
| Non-white | 23/89 | 25.8 | (ref) | 4/89 | 4.5 | (ref) | |||
| Educational background | None to 8th grade | 20/77 | 26.0 | 1.09 (0.44–2.71) | 0.850 | 4/77 | 5.2 | * | 0.302 |
| High school and university | 9/37 | 24.3 | (ref) | 0/37 | 0.0 | (ref) | |||
| Income | No income | 26/97 | 26.8 | 2.93 (0.63–13.63) | 0.154 | 2/97 | 2.1 | 0.19 (0.03-1.23) | 0.115 |
| With income | 2/18 | 11.1 | (ref) | 2/18 | 11.1 | (ref) | |||
| Assistance by Psychosocial Care Centers (CAPS) | Yes | 4/29 | 13.8 | 0.39 (0.12–1,26) | 0.108 | 2/29 | 6.9 | 3.00 (0.44-20.35) | 0.260 |
| No | 25/87 | 28.7 | (ref) | 2/87 | 2.3 | (ref) | |||
| Use of licit and/or illicit drugs | Yes | 22/87 | 25.3 | 1.06 (0.40–2.83) | 0.901 | 3/87 | 3.4 | 1.00 (0.11-9.24) | 0.740 |
| No | 7/29 | 24.1 | (ref) | 1/29 | 3.4 | (ref) | |||
| Alcohol consumption | Yes | 16/62 | 25.8 | 1.09 (0.47–2.55) | 0.830 | 2/62 | 3.2 | 0.87 (0.13–5.97) | 0.637 |
| Tobacco use | Yes | 8/32 | 25.0 | 1.00 (0.39–2.56) | 1.000 | 2/32 | 6.2 | 0.96 (0.10–8.84) | 0.725 |
| Cocaine use | Yes | 4/31 | 12.9 | 0.36 (0.11–1.12) | 0.069 | 1/30 | 3.3 | 2.62 (0.39–17.85) | 0.305 |
| Marijuana use | Yes | 6/30 | 20.0 | 0.68 (0.25–1.89) | 0.463 | 0/31 | 0.0 | * | 0.283 |
| Crack use | Yes | 3/14 | 21.4 | 0.79 (0.08–6.91) | 0.742 | 0/14 | 0.0 | * | 0.593 |
| Other drugs | Yes | 1/5 | 20.0 | 0.74 (0.08–6.91) | 0.792 | 0/5 | 0.0 | * | 0.836 |
| City of origin | Others | 25/80 | 31.3 | 3.52 (1.12–11.05) | 0.043 | 4/80 | 5.0 | * | 0.394 |
| São Paulo | 4/35 | 11.4 | (ref) | 0/35 | 0.0 | (ref) | |||
| Shelter type | Hostel | 14/64 | 21.9 | 0.69 (0.29–1.60) | 0.389 | 4/64 | 6.2 | * | 0.089 |
| Street | 9/28 | 32.1 | 1.61 (0.63–4.11) | 0.316 | 0/30 | 0.0 | * | 0.326 | |
| Causes for becoming homeless | |||||||||
| Family conflicts | Yes | 6/47 | 12.8 | (ref) | 0.035 | 1/64 | 1.6 | 0.23 (0.02–2.31) | 0.213 |
| No | 19/64 | 29.7 | 2.88 (1.05–7.93) | 3/47 | 6.4 | (ref) | |||
| Unemployment | Yes | 8/33 | 24.2 | 1.15 (0.44–3.00) | 0.778 | 1/33 | 3.0 | 0.79 (0.08–7.30) | 0.657 |
| No | 17/78 | 21.8 | (ref) | 3/78 | 3.8 | (ref) | |||
| Alcohol and drugs | Yes | 5/26 | 19.2 | 0.77 (0.26–2.32) | 0.646 | 2/26 | 7.7 | 3.27 (0.48–22.08) | 0.233 |
| No | 20/85 | 23.5 | (ref) | 2/85 | 2.4 | (ref) | |||
| Another motive | Yes | 5/18 | 27.8 | 1.40 (0.45–4.41) | 0.560 | 1/18 | 5.6 | 1.72 (0.19–15.64) | 0.512 |
| No | 20/93 | 21.5 | (ref) | 3/93 | 3.2 | (ref) | |||
| Housing loss | Yes | 4/12 | 33.3 | 1.86 (0.51–6.77) | 0.342 | 0/12 | 0.0 | * | 0.629 |
| No | 21/99 | 21.2 | (ref) | 4/99 | 4.0 | (ref) | |||
CMIA-reagent samples for anti-T. pallidum antibodies were confirmed by VDRL non-treponemal test. VDRL non-reagent samples were confirmed by treponemal rapid immunochromatographic test. A rapid immunoblot assay confirmed seropositivity to HIV. Overall, anti-T. pallidum antibodies were observed in 29/116 (25.0%), anti-HCV antibodies in 4/116 (3.4%), and anti-HIV antibodies in 2/116 (1.7%) individuals, both co-infected with T. pallidum (Table 2).
Results of anti-Treponema pallidum, anti-HCV and anti-HIV antibodies in homeless people of São Paulo city, Brazil.
Associated risk factors for syphilis exposure in homeless were to be born or had previously lived in another city (p = 0.043) and to have become homeless due to family conflicts (p = 0.035). Other variables such as sex (p = 0.234), pregnancy (p = 0.410), marital status (p = 0.360), racial self-declaration (p = 0.704), educational background (p = 0.850), income (p = 0.154), assistance by psychosocial care centers (CAPS) (p = 0.108), use of licit and/or illicit drugs (p > 0.05), use of shelter (hostels, street, occupancy) (p > 0.05) were not statistically significant (Table 1). There were no risk factors significantly associated with the presence of anti-HCV antibodies (p > 0.05) (Table 1). Risk factors associated with HIV could not be analyzed due to the low HIV seropositive rate.
DiscussionTo the authors’ knowledge, the seroprevalence of anti-T. pallidum antibodies assessed herein (25.0%) is the highest in homeless persons worldwide, which ranges from 3/569 (0.5%) in Iran,13 5/175 (2.9%) in Kenya,14 22/554 (4.0%) in India,15 to 18/132 (13.6%) in the USA.16 Additionally, syphilis was detected in 19/330 (5.7%) homeless persons in 2002-2003,17 and in 97/1,391 (7.0%) in 2006-2007,2 both in São Paulo city, which is about 4-fold lower than the rate in present study. Five surveys have found a higher prevalence of syphilis, in other vulnerable populations, including 141/450 (31.3%) prisoners in Ethiopia,18 82/222 (36.9%) sex workers in Brazil,19 273/598 (45.6%) in Argentina 20 and 51.1% (1,010/1,978) in Rwanda,21 and 269/529 (50.8%) refugees in Italy.22
In the present study, seropositivity of anti-T. pallidum antibodies among homeless persons was associated with city of birth or previously living in a city other than Sao Paulo (p = 0.043) and had become homeless due to family conflicts (p = 0.035). In São Paulo city, most homeless persons are migrants or refugees17 and with broken or fragile family bonds,9 corroborating the findings of a previous study with migrant workers in Eastern China, which reported higher seroprevalence of anti-T. pallidum antibodies among migrants with multiple sex partners and being divorced or widowed.23 A retrospective case-control study in China with 17,585 inpatients screened for syphilis infection by serological tests, T. pallidum exposure was also associated to migration between cities.24 No risk factors for HCV exposure among homeless persons were significantly associated in the present study. Future investigations should be conducted to fully ascertain risk factors for HCV and HIV coinfection in homeless persons.
Besides homeless vulnerability, worldwide shortage of benzathine penicillin supply, the drug of choice for active syphilis treatment, may have also impacted disease spreading at the time.25 Not surprisingly, the Brazilian epidemic of syphilis contrasts with other Latin American countries, which have moved towards syphilis eradication.26,27 Other aspects may have also contributed to increase syphilis rates, including greater access to syphilis testing through rapid testing in primary health care services.25 The detection rate of acquired syphilis in Brazil increased from 2.1/100,000 in 2010 to 34.1/100,000 in 201528 and to 75.8 100,000 inhabitants in 2018.29
According to the recent national guidelines for management of sexually transmitted infections, the diagnosis of syphilis should incorporate clinical history, history of previous treatment, symptoms, in addition to results of treponemal and non-treponemal tests.11 As limitation, VDRL reagent results may represent early syphilis in recent infection or untreated late syphilis, also in activity. Therefore, the prevalence of syphilis in activity herein may be underestimated. Further studies should also consider molecular diagnosis, particularly due to higher sensitivity in primary syphilis, associated with clinical signs such as exanthema and ulcers.30
In the present study, 2/116 (1.7%) individuals have shown anti-HIV antibodies. Herein, a 4th generation test (CMIA) was used as screening test and confirmed by a 2nd generation test (immunoblot assay), according to a recognized algorithm of the Brazilian Ministry of Health.10 Also, anti-HCV antibodies were detected in 4/116 individuals by CMIA, as recommended by Brazilian Ministry of Health.11 Although the Ministry of Health has also recommended molecular testing for HIV and HCV clinical cases, the present study focused on the epidemiological approach of viral exposure rather than viral load, prognosis and treatment.
In summary, the highest worldwide syphilis prevalence to date found in the present study indicates multiple preventable causes, which may profoundly impact homeless persons' health and wellbeing. More critical, strongly associating syphilis to homeless vulnerability and lack of preventive measures and treatment. Further studies should be conducted to fully establish risk factors for sexually transmitted infections exposure in homeless people.
This research has been partially supported by the Veterinary Science Graduate Program at the Federal University of Paraná, Clinical Analysis Laboratory Unit, Clinics Hospital (UFPR), Institute of Biotechnology (UNESP), Department of Veterinary Hygiene and Public Health (UNESP), and Purdue University. The authors are thankful to Dr. Mara Lúcia Gravinatti, Dr. Gabriela Hartmann, Dr. Daniela Patricia Tozetto and the Social Center "Our Lady of Good Delivery" for helping with collection, sampling, and follow-up information.