The prevalence of nontuberculous mycobacterial (NTM) infection worldwide has increased, and lung is the most heavily affected organ.1 Because NTM and Mycobacterium tuberculosis (MTB) are both acid-fast bacilli (AFB), it is difficult to distinguish them using the AFB test alone, and drug treatment for NTM is totally different from anti-tuberculosis (TB) therapy. There is a high incidence of pulmonary TB in China,2 but Chinese hospitals still use positive AFB test, clinical symptoms, and chest radiographic abnormalities as the key diagnostic criteria for pulmonary TB. Therefore, there is a high risk of NTM pulmonary infection being misdiagnosed and incorrectly treated.3 In order to investigate the prevalence, clinical characteristics and risk factors of NTM infection in patients who met Chinese pulmonary TB diagnostic criteria, we performed this study in Zhejiang Province, southeastern China.
This investigation was conducted prospectively in sentinel sites of 12 counties in Zhejiang from January 2011 to December 2013. All the sentinel sites are part of a laboratory and hospital network, the Chinese Demonstration Zone of the prevention and treatment of infectious diseases, which was set for systematic surveillance of pulmonary TB.3 The laboratories and hospitals collected the sputum samples and clinical data of HIV negative suspected pulmonary TB patients and performed mycobacterial culture and AFB test. The AFB-positive isolates were sent to central laboratory, and first tested using a CapitalBio Mycobacterium identification microarray.3,4 The identified NTM strains were then further tested by sequencing of 16S rRNA, heat shock protein 65 (hsp65), and the RNA polymerase beta-subunit-encoding (rpoB) genes and then compared by BLAST analysis. NTM pulmonary disease was diagnosed based on criteria proposed by the American Thoracic Society (AST) guidelines.5
SPSS version 19.0 was used for the statistical analyses. The Cochran-Armitage trend test was used to evaluate the annual incidence of NTM pulmonary isolates. The distributions of smoking, alcohol abuse, bronchiectasis, pulmonary emphysema, chronic bronchitis, and living and working conditions between males and females were compared using the χ2 test. The independent risk factors affecting patient outcome was identified by logistic regression analysis including all patients. Results were considered statistically significant when p-value <0.05.
A total of 1953 AFB-positive mycobacterial isolates were cultured from the sputum samples of 1831 suspected pulmonary TB patients, whereas 113 NTM strains from 100 patients were identified. Eleven patients met the AST bacteriological criteria for NTM pulmonary disease; three patients had no clinical symptoms or chest radiography abnormality, and their isolates might have been contaminants or transient colonization; other 86 patients had positive clinical symptoms or chest radiography abnormalities, though the number of respiratory isolates were insufficient. Nineteen patients had repeatedly negative AFB tests of their sputum but had positive culture results, none of them met NTM pulmonary disease diagnostic criteria.
Microarray biochip identification showed that there were 55 Mycobacterium intracellulare and eight Mycobacterium avium isolates, 25 Mycobacterium kansasii, one Mycobacterium malmoense/szulgai, 16 Mycobacterium abscessus/chelonei, one Mycobacterium gilvum, four Mycobacterium fortuitum, and three NTM without species identification. Direct sequencing were performed in 106 NTM isolates, and out of those six isolates were not consistent with biochip identification results, including three Mycobacterium marinum, one Mycobacterium sp. JDM601, one Mycobacterium smegmatis, and one M. gilvum.
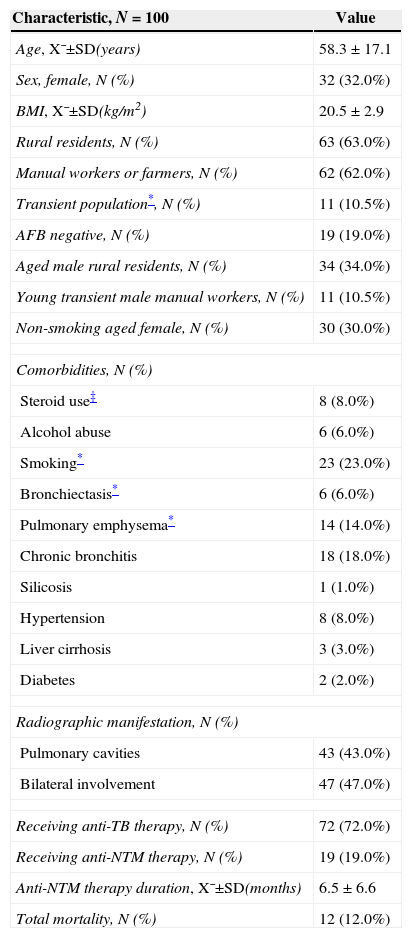
The patient clinical characteristics are shown in Table 1. Multiple logistic regression analysis determined that history of steroid use to be the only independent risk factor affecting patient prognosis. Of the eight patients using steroids, five received anti-NTM therapy while the other three died. The local hospitals did not report the indications or details for the steroid treatment, making it difficult to explain this risk factor, which may be associated with the therapy used for end-stage pneumonia patients.
Patient clinical characteristics.
| Characteristic, N=100 | Value |
|---|---|
| Age, X¯±SD(years) | 58.3±17.1 |
| Sex, female, N (%) | 32 (32.0%) |
| BMI, X¯±SD(kg/m2) | 20.5±2.9 |
| Rural residents, N (%) | 63 (63.0%) |
| Manual workers or farmers, N (%) | 62 (62.0%) |
| Transient population*, N (%) | 11 (10.5%) |
| AFB negative, N (%) | 19 (19.0%) |
| Aged male rural residents, N (%) | 34 (34.0%) |
| Young transient male manual workers, N (%) | 11 (10.5%) |
| Non-smoking aged female, N (%) | 30 (30.0%) |
| Comorbidities, N (%) | |
| Steroid use‡ | 8 (8.0%) |
| Alcohol abuse | 6 (6.0%) |
| Smoking* | 23 (23.0%) |
| Bronchiectasis* | 6 (6.0%) |
| Pulmonary emphysema* | 14 (14.0%) |
| Chronic bronchitis | 18 (18.0%) |
| Silicosis | 1 (1.0%) |
| Hypertension | 8 (8.0%) |
| Liver cirrhosis | 3 (3.0%) |
| Diabetes | 2 (2.0%) |
| Radiographic manifestation, N (%) | |
| Pulmonary cavities | 43 (43.0%) |
| Bilateral involvement | 47 (47.0%) |
| Receiving anti-TB therapy, N (%) | 72 (72.0%) |
| Receiving anti-NTM therapy, N (%) | 19 (19.0%) |
| Anti-NTM therapy duration, X¯±SD(months) | 6.5±6.6 |
| Total mortality, N (%) | 12 (12.0%) |
The prevalence of pulmonary TB cases in the 12 counties was 62.1 per 100,000 in 2011, 68.6 per 100,000 in 2012, 59.2 per 100,000 in 2013. As the local population was close to 71.8 million, the annual incidence of NTM infected patients and NTM pulmonary diseases could be estimated. In 2011 they were 3.07 and 0.43 per 100,000, in 2012 were 3.92 and 0.48 per 100,000, in 2013 were 3.36 and 0.17 per 100,000. The differences of annual incidence were not statistically significant (p>0.05).
In conclusion, we found that there was a high possibility that NTM pulmonary infected patient might be misdiagnosed as pulmonary tuberculosis by Chinese pulmonary tuberculosis diagnostic criteria. In addition, aged male rural residents, young male manual transient workers, and aged non-smoking female were three distinct patient groups. Physicians should differentiate NTM pulmonary disease carefully from pulmonary TB.
FundingThis study was funded by the National Scientific and Technological Major Project of China (Grant Nos. 2011ZX10004-901, 2013ZX10004-904, 2014ZX10004-008), and the Fundamental Research Funds for the Central Universities.
Conflicts of interestThe authors declare no conflicts of interest.