Several studies have found that HIV+ people are at increased risk for premature cardiovascular disease and endothelial damage compared with HIV− population.1 As for the general population also for HIV+ patients high blood pressure (BP) is one of the most preventable causes of cardiovascular disease morbidity and mortality. In recent years, attention has focused on anti-hypertensive (AH) drugs that block the renin–angiotensin system, because these drugs have a multiple favourable effect on mortality and morbidity. Specifically telmisartan showed agonistic effect on PPAR gamma. This finding is particularly relevant in HIV+ patients who have higher incidence of metabolic disorder related with HIV infection and combined antiretroviral therapy (cART). In HIV population limited data showed the effect of AH therapy and some trials with telmisartan are available in this population.2–4 Moreover in studies involving HIV− patients it was previously demonstrated that telmisartan had the higher long term efficacy. The aim of this study was to evaluate the long term effect of AH therapy with telmisartan over a period of 144 weeks in HIV+ patients by the means of both high blood pressure control and amelioration of metabolic abnormalities.
This was a prospective observational study involving 13 Caucasian male HIV+ patients having been in cART and newly diagnosed with high blood pressure enrolled from July 2009 to July 2010 and longitudinally followed for 3 years.
All patients were treated with telmisartan at the dose of 80mg administered orally once a day and were controlled every 6 month for 3 years. Participants were asked to adhere to their usual diet and lifestyle during the study.
In 144 weeks of treatment we did not observe any adverse event, or drop out and adherence to antihypertensive therapy as well as that to the cART was complete.
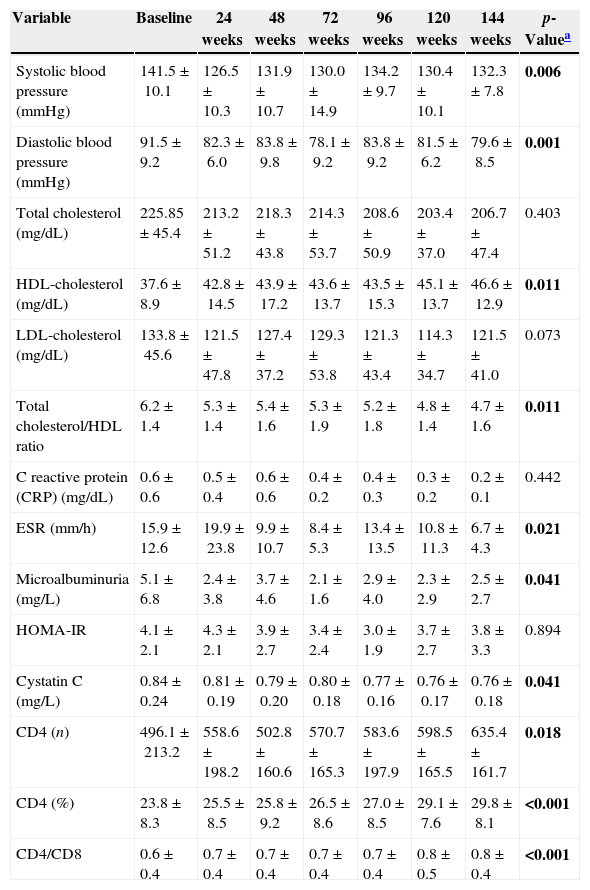
Analysis of the data has shown a clear improvement in blood pressure, the difference between the baseline value and week 144 was 9.2±3.4mmHg (p=0.006) for Systolic BP and 11.9±2.9mmHg (p=0.001) for Diastolic BP. We also observed an improvement of the main metabolic parameters, in particular HDL-cholesterol significantly increased between baseline and 144 weeks (p=0.011; delta T0–T144=9.0±2.3), with a statistically significant reduction of the ratio cholesterol total/HDL (p=0.011). A significant reduction of triglycerides was also observed during the follow-up period (p=0.021). Total cholesterol and LDL-cholesterol also decreased, although this finding did not reach the statistical significance.
In addition we observed an improvement of the main inflammatory markers with a significant reduction of ESR (p=0.021), a significant reduction of microalbuminuria (p=0.041) and a significant reduction of cystatin-C (p=0.041); finally CRP also decreased but the differences was not statistically significant (Table 1).
Mean±standard deviation of parameters according to different phase of the follow-up.
| Variable | Baseline | 24 weeks | 48 weeks | 72 weeks | 96 weeks | 120 weeks | 144 weeks | p-Valuea |
|---|---|---|---|---|---|---|---|---|
| Systolic blood pressure (mmHg) | 141.5±10.1 | 126.5±10.3 | 131.9±10.7 | 130.0±14.9 | 134.2±9.7 | 130.4±10.1 | 132.3±7.8 | 0.006 |
| Diastolic blood pressure (mmHg) | 91.5±9.2 | 82.3±6.0 | 83.8±9.8 | 78.1±9.2 | 83.8±9.2 | 81.5±6.2 | 79.6±8.5 | 0.001 |
| Total cholesterol (mg/dL) | 225.85±45.4 | 213.2±51.2 | 218.3±43.8 | 214.3±53.7 | 208.6±50.9 | 203.4±37.0 | 206.7±47.4 | 0.403 |
| HDL-cholesterol (mg/dL) | 37.6±8.9 | 42.8±14.5 | 43.9±17.2 | 43.6±13.7 | 43.5±15.3 | 45.1±13.7 | 46.6±12.9 | 0.011 |
| LDL-cholesterol (mg/dL) | 133.8±45.6 | 121.5±47.8 | 127.4±37.2 | 129.3±53.8 | 121.3±43.4 | 114.3±34.7 | 121.5±41.0 | 0.073 |
| Total cholesterol/HDL ratio | 6.2±1.4 | 5.3±1.4 | 5.4±1.6 | 5.3±1.9 | 5.2±1.8 | 4.8±1.4 | 4.7±1.6 | 0.011 |
| C reactive protein (CRP) (mg/dL) | 0.6±0.6 | 0.5±0.4 | 0.6±0.6 | 0.4±0.2 | 0.4±0.3 | 0.3±0.2 | 0.2±0.1 | 0.442 |
| ESR (mm/h) | 15.9±12.6 | 19.9±23.8 | 9.9±10.7 | 8.4±5.3 | 13.4±13.5 | 10.8±11.3 | 6.7±4.3 | 0.021 |
| Microalbuminuria (mg/L) | 5.1±6.8 | 2.4±3.8 | 3.7±4.6 | 2.1±1.6 | 2.9±4.0 | 2.3±2.9 | 2.5±2.7 | 0.041 |
| HOMA-IR | 4.1±2.1 | 4.3±2.1 | 3.9±2.7 | 3.4±2.4 | 3.0±1.9 | 3.7±2.7 | 3.8±3.3 | 0.894 |
| Cystatin C (mg/L) | 0.84±0.24 | 0.81±0.19 | 0.79±0.20 | 0.80±0.18 | 0.77±0.16 | 0.76±0.17 | 0.76±0.18 | 0.041 |
| CD4 (n) | 496.1±213.2 | 558.6±198.2 | 502.8±160.6 | 570.7±165.3 | 583.6±197.9 | 598.5±165.5 | 635.4±161.7 | 0.018 |
| CD4 (%) | 23.8±8.3 | 25.5±8.5 | 25.8±9.2 | 26.5±8.6 | 27.0±8.5 | 29.1±7.6 | 29.8±8.1 | <0.001 |
| CD4/CD8 | 0.6±0.4 | 0.7±0.4 | 0.7±0.4 | 0.7±0.4 | 0.7±0.4 | 0.8±0.5 | 0.8±0.4 | <0.001 |
Throughout the course of the trial the CD4 absolute number and CD4 percentage significantly improved (p=0.018 and p<0.001, respectively); with significant improvement of CD4/CD8 ratio between 0.6±0.4 to 0.8±0.4 (p<0.001) at 144 weeks and the remarkable no virological failure (HIV-RNA >400cp/mL) was observed in patients during the follow-up period (Table 1). In conclusion telmisartan was effective in improving hypertension for 144 weeks. It determined the improvement of lipid profile, cystatin C and microalbuminuria, markers of renal function and cardiovascular risk.5 Telmisartan does not interfere with cART, not interfering with the recovery immunological in HIV+ patients. Telmisartan, thus, has a long term efficacy and excellent tolerability. Therefore it should be considered the first choice in the treatment of hypertension in HIV+.
Conflicts of interestThe authors declare no conflicts of interest.