Describe the diagnostic characteristics of a conventional multiplex PCR for the diagnosis of S. stercoralis, N. americanus and Ancylostomas spp.
MethodsFecal samples were collected from a cross-sectional study in Orán department, Salta province, Argentina. The stool samples were analyzed using concentration-sedimentation, Harada Mori, McMaster, and Baermann techniques. DNA was extracted from 50 mg fecal sample using the FastPrep® Spin Kit for Soil. Three pairs of primers were used for the amplification of three products of 101, 330, and 577 base pairs (bp) for S. stercoralis, N. americanus and Ancylostoma spp, respectively. The sensitivity and analytical specificity of multiplex PCR were evaluated, as well as the sensitivity and diagnostic specificity, using a composite standard and Bayesian approach.
Results and ConclusionsMultiplex PCR did not present cross-reaction with other intestinal parasites, and the detection limit for multiplex PCR was between 2 and 20 pg of genomic DNA. In addition it presented a diagnostic sensitivity of 97.4% for S. stercoralis and 90.3% for hookworms with a specificity of 100% and 87.6%, respectively. PCR identified a higher proportion (p <0.01) of coinfections (15.3%) than microscopic techniques (3.5%). Also, multiplex PCR showed that there was a positive association between S. stercoralis and hookworms (odds ratio = 2.12). However, this association was due to N. americanus (odds ratio= 3.22), since no association was observed between S. stercoralis and Ancylostoma spp. Neither was an association observed between the two species of hookworms.
Soil-transmitted helminths (STH) are a major public health problem worldwide, being the most prevalent neglected tropical disease in many poor communities worldwide.1 STH infections are caused by different species of parasitic worms. Strongyloides stercoralis and the hookworms Necator americanus and Ancylostoma duodenale are STH of significant clinical relevance. Hookworms cause morbidity with chronic intestinal blood loss, iron deficiency anemia, and protein malnutrition affecting growth and cognitive development in children,2 while S. stercoralis, through its ability for autoinfection, can generate chronic infections that can last a lifetime.3 However, the morbidity of S. stercoralis remains unclear.4
Mixed infections between hookworms and S. stercoralis are common in endemic areas.5 This may be because both share the same infection route.6 However, these parasites have different life cycles, and while the diagnosis of S. stercoralis depends on the identification of larvae, the diagnosis of hookworm, and other STH, is mainly based on the identification of eggs. The Kato-Katz method, which is based on egg quantification, is the most commonly used technique for assessing STH prevalence and intensity of infection in epidemiological surveys.7 Other methodologies include the concentration-sedimentation technique, and Mc Master, which, like Kato-Katz, are cheap and simple methods. However, the recommended diagnostic methods for S. stercoralis are the Baermann, agar plate, and Harada-Mori techniques, since they allow visualization of the larvae. These methods are laborious and require specialized technicians who can morphologically differentiate S. stercoralis larvae from hookworm larvae.8 Therefore, such methods are not suitable for routine use in many laboratories. In turn, the diagnosis of hookworms is not as challenging as S. stercoralis. However, microscopy of fecal samples should be done rapidly because hookworm eggs disintegrate in fecal matter,9 and the identification of the different hookworm species cannot be done through the observation of eggs. These difficulties may underestimate the true prevalence of these parasites and the prevalence of mixed infections.
Interventions for the control of STH require a sound and timely assessment of the epidemiological situation based on sensitive and specific diagnostic methods. Herein we describe a conventional multiplex PCR assay, which can simultaneously detect three species of STH, namely N. americanus, Ancylostoma spp. (including A. caninum, A. ceylanicum and A. duodenale), and S. stercoralis, which as we have recently shown, correlate with their distribution.10
Material and methodsStudy populationA sample size of 258 individuals was calculated using Burderer's formula,11 assuming a prevalence of 30% for S. stercoralis,12 an anticipated PCR sensitivity of 72%,13 an absolute precision of 10%, and a confidence interval of 95%.
Fecal samples were collected from a cross-sectional study in Orán department, Salta province, Argentina. Eligible individuals were children and adults assigned to a health intervention program carried out by the teams of the Universidad Nacional de Salta.
The study was approved by the Bioethics Committee of the Faculty of Health Sciences at the Universidad Nacional de Salta (Res-CDN° 417/15). All participants were informed about the purpose and procedures of the study. All individuals infected with STH were treated with a combination of single dose albendazole (400 mg) and ivermectin (200 μg/kg).
MicroscopyA single stool sample was collected from each participant in sterile containers without preservatives. Stool samples were dated, coded and then analyzed within 24 hours of collection using concentration-sedimentation and McMaster techniques for egg detection and Baermann and Harada-Mori techniques for larvae detection.14 Any sample that tested positive for at least one technique was considered positive for that particular species of STH.
DNA extraction and PCRDNA was extracted from 50 mg fecal sample using the FastPrep® Spin Kit for Soil (MP Biomedicals, Santa Ana, USA) according to the manufacturer's instructions for all parasites.15 All samples were processed in Salta, Argentina.
Specific primers for detecting N. americanus and S. stercoralis were designed by other authors.16,17 The primers for detecting Ancylostoma spp were designed using Primer3 (v.0.4.0) software18 from ribosomal DNA region of A. duodenale (GenBank Accession N°: EU344797.1). The forward primer was CAT GAA TGC CGC CTT ACT GC and the reverse primer was AAG TTC AGC GGG TAG TCA CG. The three primer pairs allow the amplification of three products of 101, 330 and, 577 base pairs (bp) for S. stercoralis, N. americanus and Ancylostoma spp, respectively.
PCR reaction mixture consisted of 10 µl DNA template, 0.125 µl GoTaq® polymerase (Promega, Madison, USA), 5 µl 10x GoTaq® buffer (Promega, Madison, USA), 0.5 µl dNTP (10 mM), 0.2 µM N. americanus primers, 0.2 µM S. stercoralis primers, 0.3 µM Ancylostoma spp primers, and 5.875 µl MiliQ water. PCR conditions consisted of an initial denaturation at 95° C for 3 min, followed by 35 cycles of 95° C for 45 s, 61° C for 1 min, and 72° C for 45 s, and a final elongation was performed at 72° C for 5 min. For each PCR, a negative control (MiliQ water) was run together. PCR products were analyzed by electrophoresis on 1.5% agarose gels, stained with GelRed® 1X (Biotium, San Francisco, USA), and visualized on a UV transilluminator.
Sequencing of PCR productsFive PCR products from each helminth were randomly chosen. The PCR products were recovered from the 1.5% agarose gels using the Puro Gel Extraction kit (PB-L Productos Bio-Logicos, Argentina) and then sequenced at the Instituto Nacional de Tecnología Agropecuaria (INTA, Castelar).
The sequences obtained were aligned with the MEGA X software,19 and an alignment with the NCBI database was performed with the BLAST software.20
Analytical specificity and limit of detection of multiplex PCRThe multiplex PCR was evaluated for specificity using fecal samples with humam intestinal parasites such as Ascaris lumbricoides, Trichuris trichiura, Giardia intestinalis, Hymenolepis diminuta, Hymenolepis nana, Enterobius vermicularis, and Entamoeba coli. In turn, to determine the limit of DNA detection, 10-fold serial dilutions of a mixture of three positive samples of S. stercoralis, N. americanus, and Ancylostoma spp were performed. The mixture of DNA from Ancylostoma spp., N. americanus, and S. stercoralis was performed by measuring the concentration of each DNA extraction individually with the NanoDrop 2000 spectrophotometer (Thermo Fisher, Waltham, United States). Each DNA extraction was then diluted to a concentration of 60 ng/µl with ultrapure water and mixed in equal parts, obtaining a final concentration of 20 ng/µl of each DNA extract in the mixture.
Statistical analysisDiagnostic precision parameters for multiplex PCR and the combination of the four microscopic techniques were evaluated by two different approaches. First, multiplex PCR was compared with a composite standard, consisting of the result of multiplex PCR and microscopic techniques. In this case, an individual was considered positive if the presence of an egg or larva was observed by any of the microscopic techniques and/or multiplex PCR. All these analyses were carried out with R software,21 and statistical significance was assessed by McNemar test. In the second approach, since microscopic methods are imperfect standards due to false negative results and less than perfect sensitivity and specificity, a Bayesian approach was used to estimate diagnostic parameters in the absence of a gold standard. It was assumed that PCR follows a biological process that is different from microscopic techniques,22 and model 101 of the online platform Modeling of Infectious Disease Center (MICE) was used.23 For this, the sample was randomly divided into two subpopulations with 65% of the data in one and 35% in the other. In this case, a significant difference was considered when there was no overlap of the confidence intervals.
To measure the correlation between S. stercoralis and hookworms, logistic regression was performed with the R software.21
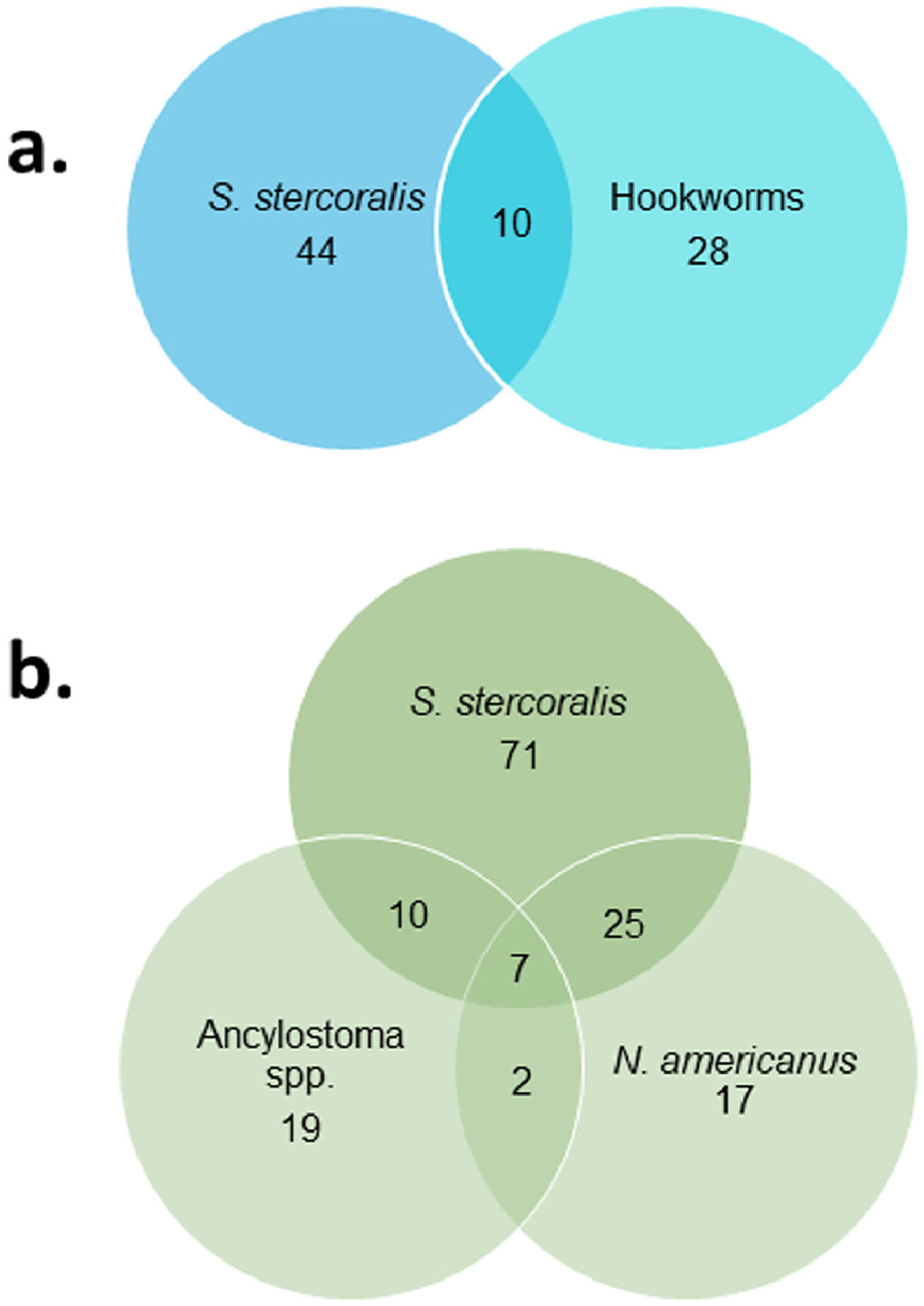
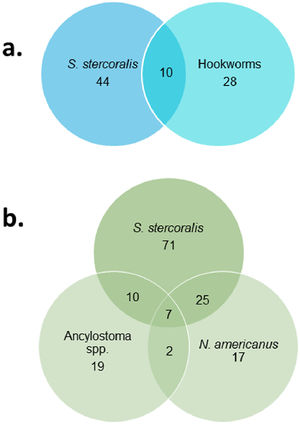
ResultsMixed infections and asociation between helminth speciesOut of 287 stool samples collected in the province of Salta, 13% (38/287) and 19% (54/287) were positive for hookworms and S. stercoralis by microscopic techniques, respectively. Multiplex PCR were positive in 28% (80/287) and 39% (113/297) for hookworms and S. stercoralis, respectively (Fig. 1). In addition, multiplex PCR identified a higher proportion (p <0.01) of coinfections (15.3%; 44/287) than microscopic techniques (3.5%; 10/287).
There was a positive association between S. stercoralis and hookworms (odds ratio= 2.12 CI 95%: 1.25 – 3.58) with multiplex PCR. However, this association was due to N. americanus (odds ratio= 3.22 CI 95%: 1.72 – 6.04), since no association was observed between S. stercoralis and Ancylostoma spp. (odds ratio= 1.29 CI 95%: 0.65 – 2.57). Neither was an association observed between the two species of hookworms (odds ratio= 1.53 CI 95%: 0.67 – 3.47). On the other hand, no significant association was observed between S. stercoralis and hookworms when analyzing the results of microscopic tests.
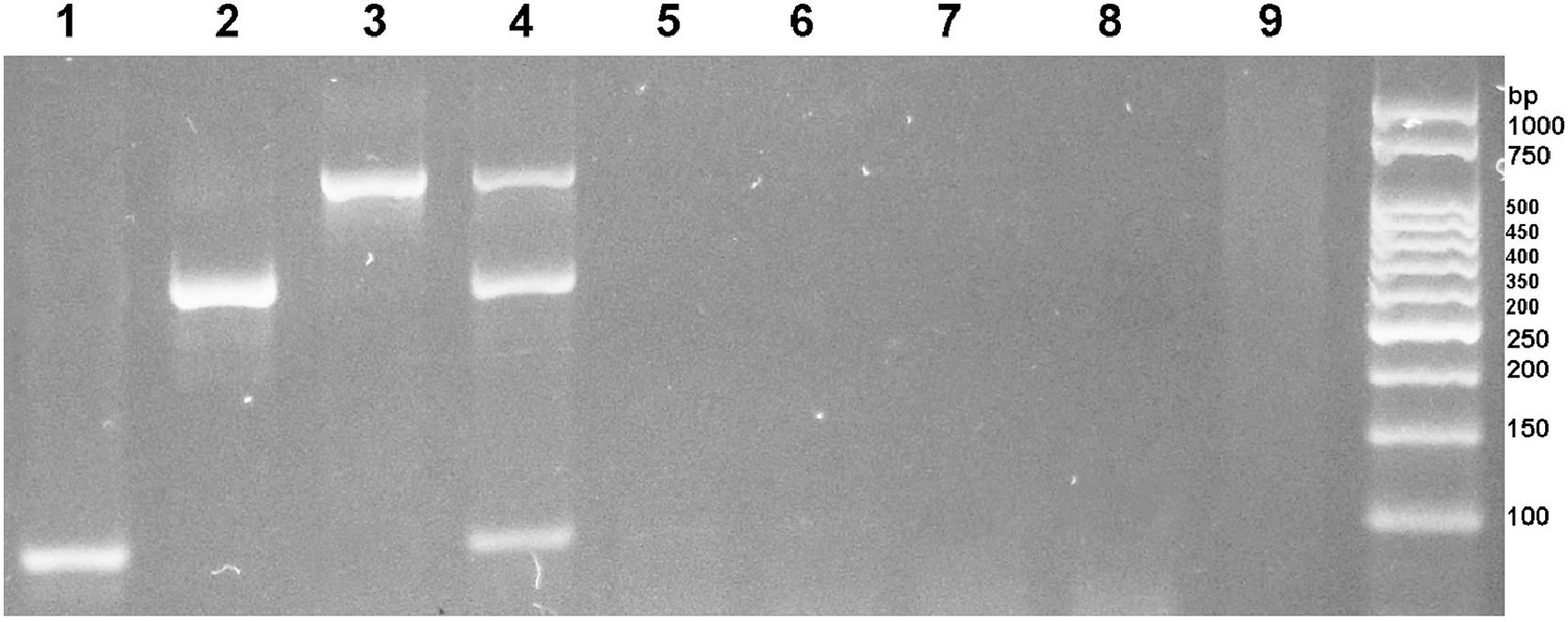
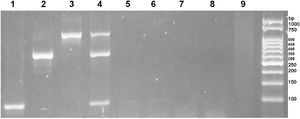
Sensitivity and analytical specificity of multiplex PCRMultiplex PCR for the diagnosis of Ancylostoma spp., N. americanus, and S. stercoralis was able to specifically amplify the DNA of the three helminths with no cross-reaction with other intestinal parasites (Fig. 2). In addition, the sequences analyses of the PCR products revealed that the PCR products of S. stercoralis showed 100% homology with the 18S sequence of S. stercoralis (GenBank: AF279916), the PCR products of N. americanus presented two different sequences that presented 100% and 98.9% homology with the sequence ITS1 of N. americanus (GenBank: AJ001680.1), and the PCR products of Ancylostoma spp presented two different sequences, one presented 100% homology with the ribosomal sequences of A. duodenale (GenBank: EU344797.1) and the other 99.8% homology with A. duodenale (GenBank: EU344797.1 and MK271367.1), 98.7% with A. caninum (GenBanK: KP844730.1), and 98.5% with A. ceylanicum (GenBank: LC036567). This indicates that multiplex PCR is specific for S. stercoralis, N. americanus, and Ancylostoma spp.
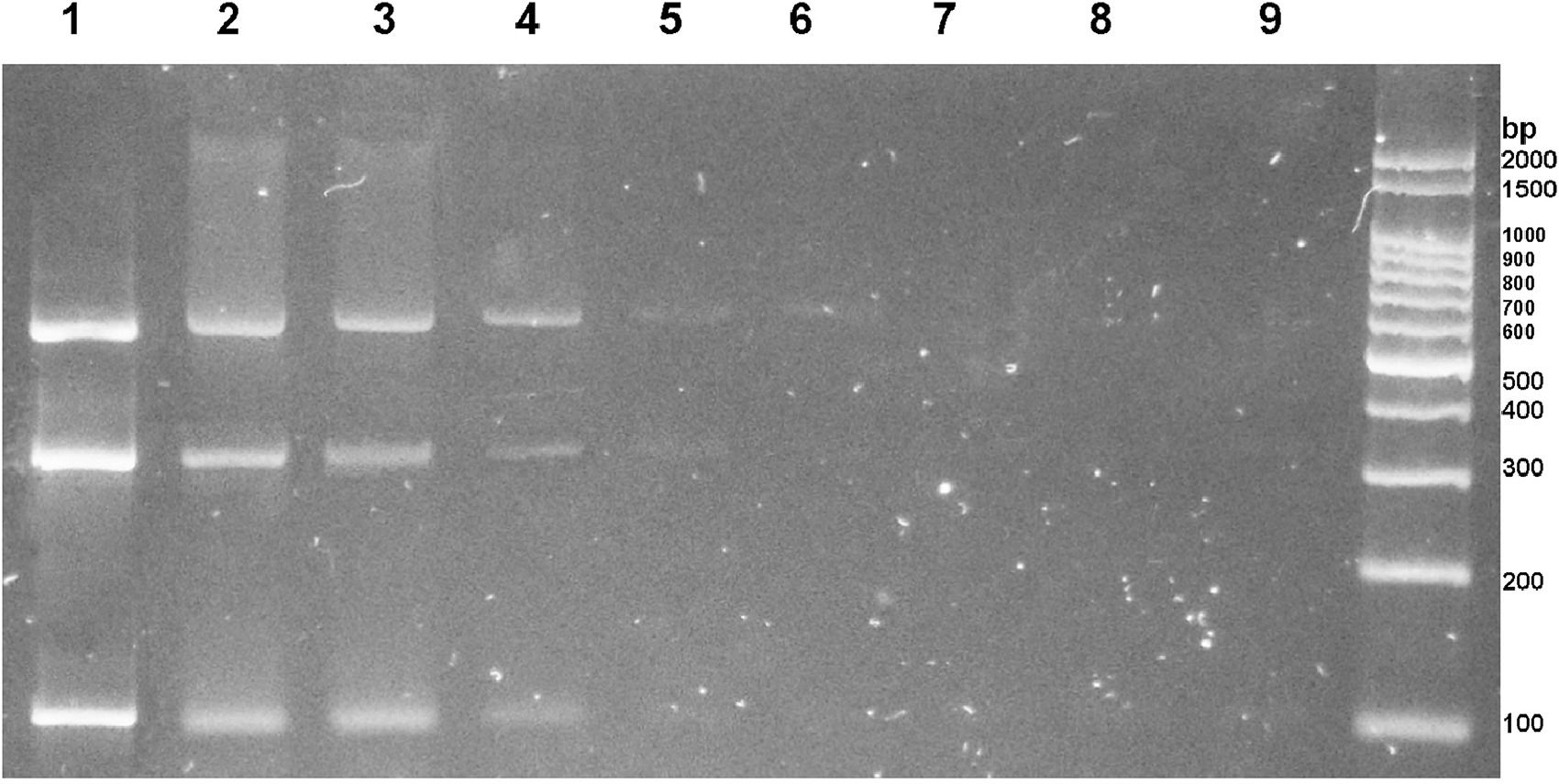
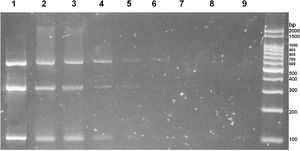
Sensitivity analysis revealed that the detection limit for multiplex PCR was between 2 and 20 pg of genomic DNA from each helminth of interest (Fig. 3).
In lanes 1, 2, and 3 fecal samples positive for S. stercoralis, N. americanus and A. duodenale, respectively. In lane 4, a positive stool for S. stercoralis, N. americanus and A. duodenale. In lanes 5, 6, 7, 8, and 9 fecal samples with A. lumbricodes, T. trichiura and H. nana, G. intestinalis and H. diminuta, E. vermicularis and E. coli, and A. lumbricodes and T. trichiura.
Successive 1/10 dilutions of a mixture of DNA from Ancylostoma spp., N. americanus, and S. stercoralis are observed in lanes 1 to 9, with an initial concentration of 20 ng/µl.
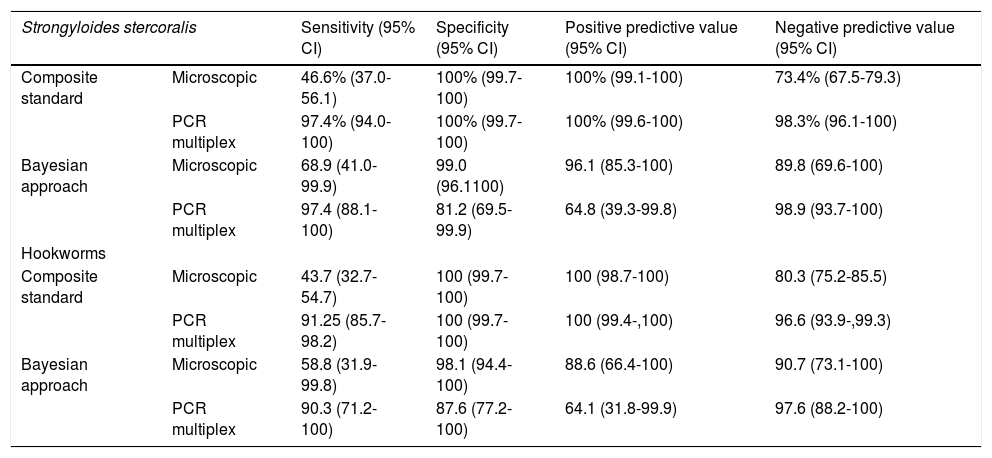
Diagnostic evaluation of multiplex PCRThe diagnostic sensitivity of multiplex PCR for S. stercoralis and hookworms was significantly higher using the composite standard (McNemar test p <0.01), and the Bayesian approach. In turn, the diagnostic specificity of multiplex PCR was not significantly different from microscopic techniques using the composite standard (McNemar test p >0.05), and the bayesian approach (Table 1).
Diagnostic sensitivity and specificity of microscopic techniques and multiplex PCR.
As deworming campaigns expand and new targets are outlined for the control of STH as a public health problem, diagnostic tools with the appropriate sensitivity, specificity and ability of species identification are needed to meet those targets.24 The lack of accurate diagnostic tools is also an important consideration for mapping the epidemiological situation of STH. Limitations in diagnostic techniques have led to the exclusion, for years, of S. stercoralis in the WHO strategies against STH. However, the new WHO report incorporates a control strategy for S. stercoralis in school-age children.24 This new scenario implies that rapid and sensitive diagnostic methods are needed to identify risk areas for S. stercoralis, which can also be risk areas for hookworms.25 Therefore, in this study, a diagnostic strategy that would allow simultaneous identification of S. stercoralis infection and the two species of hookworms was approached. Multiplex PCR was able to specifically detect the three species of interest even in the presence of other helminths with the advantage of being able to differentiate between hookworm species. This implies that the analytical specificity of the technique would not be compromised in endemic areas where different species of helminths coexist. However, the detection limit of multiplex PCR (20 pg/µl) was shown to be three orders of magnitude higher than the limit of detection (fg/µl) of multi-parallel qPCR for S. stercoralis and hookworms.15 This demonstrates a lower analytical sensitivity of conventional PCR compared to qPCR. However, it was shown that, in areas of high prevalence, conventional PCR has a higher sensitivity than microscopic techniques with the same specificity. It should be noted that in the absence of a reference technique, the Bayesian approach allows an estimation of the true sensitivity and specificity of a new diagnostic technique.23 Through this approach, it was observed that the sensitivity of multiplex PCR for S. stercoralis was higher than that reported by Buonfrate et al.13 and was similar to the sensitivity and specificity of a urine PCR technique evaluated in the same geographical area.12 On the other hand, the sensitivity and specificity of multiplex PCR for hookworms are similar to that reported for qPCR.26
In addition, a single multiplex PCR assay had a sensitivity that can only be achieved with microscopic methods if several assays are performed with numerous samples. These results show that conventional PCR provides sufficient information for estimating prevalence, and identifying priority areas for control programs, where infection levels are typically high. Nonetheless, techniques such as real-time PCR are more suitable to evaluate elimination of transmission, clinical trials, and mathematical modeling studies of transmission dynamics.27,28
Mixed infection is an important factor to consider before implementing mass drug administration, and selecting the appropriate antiparasitic drugs.29 In turn, infection with one helminth can alter the intensity of infection by another helminth.30 A significantly higher proportion of mixed infections was detected by multiplex PCR. In this study, the most frequent mixed infections were S. stercoralis and N. americanus, and S. stercoralis and Ancylostoma spp. This may be due to a higher prevalence of S. stercoralis in the area. In line with other reports,31,32 we also found a positive association between S. stercoralis and hookworms. However, this association only applied to N. americanus, and that no correlation was found between the two species of hookworms. Subsequent studies in areas with a high prevalence of hookworm and S. stercoralis are necessary to understand whether these results are specific to this area or due to distinctive features of different species of hookworms and potential interaction between the different species of hookworms. Also, the correlation analysis was significant only with the multiplex PCR results, noting that there was greater correlation between S. stercoralis and hookworms when high sensitivity diagnostic methods were used.10 At the same time, the correlation between S. stercoralis and hookworms highlights the importance of generating control strategies that include both to allow a more efficient use of resources.
Certain limitations must be taken into account when interpreting this study. First, our multiplex PCR only provides qualitative results, being unable to measure the intensity of the infection. This presents an important limitation in the case of hookworm, since the number of excreted eggs cannot be known; however, it is of no importance for S. stercoralis, whose intensity of infection cannot be clearly measured,12,33 so a quantitative measurement does not represent an advantage in the latter case. In addition, it should be noted that although there is a relationship between the qPCR result and the intensity of infection, this relationship is neither simple nor fully understood.33 Moreover, the correlation between eggs per gram and the respective qPCR results is lower in hookworms than in T. trichiura y A. lumbricoides.28 Second, it was not possible to evaluate whether the primers of the genus Ancylostoma had the ability to differentiate between Ancylostoma ceylanicum and Ancylostoma duodenale. However, as no cases of A. ceylanicum have been reported in northern Argentina,34 it is likely that all the Ancylostoma cases detected were due to A. duodenale. Further investigations will elucidate whether Ancylostoma primers can amplify the DNA of various Ancylostoma species.
Finally, it can be affirmed that multiplex PCR presented a superior performance to microscopic techniques in high prevalence areas. This is an additional tool to detect several species of parasites simultaneously in a large population and to evaluate the epidemiological situation of the region in order to design deworming strategies.
This work was supported by a research grant from CIUNSa N°2399 and Fundación Mundo Sano, Buenos Aires.