The outbreak of the new coronavirus (SARS-CoV-2) causing the coronavirus disease (COVID-19) has spread globally. As of June 18, 2020, a high maternal mortality rate due to SARS-CoV-2 infections was identified in Brazil, representing most of the world cases at that time. An observational, cross-sectional study was performed with pregnant women admitted in two maternity hospitals located in Salvador/Bahia and their newborns, from May 24th up to July 17th of 2020. Among 329 pregnant women enrolled at hospital admission, a high prevalence (n=28; 8.5%) of pregnant women with COVID-19 was observed, as well as a high proportion of asymptomatic cases (n=19; 67.9%). Two newborns had detectable SARS-CoV-2 but evolved without abnormalities. This data highlight the importance of identifying pregnant women with COVID-19 for proper isolation measures to prevent in-hospital transmission.
The Severe Acute Respiratory Syndrome caused by coronavirus 2 (SARS-CoV-2) is spreading extremely fast throughout the world, with an enormous medical burden, social and economic impact. Almost all countries were heavily affected by the infection with thousands of deaths.1 Up to March 2021, Brazil reported almost thirteen million people infected with more than 330,000 deaths distributed throughout the country,2 the world's third place in number of infection and second in number of deaths at that time. Infection affects all age groups, but pregnant women and newborns may have significantly higher morbidity and mortality.3,4 A dramatic maternal mortality rate due to COVID-19 in Brazil (12.7%) represented most of the world cases. A total of 58.9% of women who died were admitted to an intensive care unit (ICU), and above all, 22.6% did not have access to an ICU bed before dying.5
Considering the COVID-19 pandemic, the potential benefits of universal testing in pregnant women could include the ability to use COVID-19 status to improve hospital isolation practices and implementation of preventive actions.5 Herein we describe the clinical-epidemiological profile of mothers and neonates during the highest COVID-19 transmission season in Salvador.
An observational, cross-sectional study performed with pregnant women and their newborns from May 24th up to July 17th of 2020, coinciding with the first pandemic peak of COVID-19 notified cases in the city of Salvador, Brazil.6 Two maternity hospitals were involved, the Hospital Santo Amaro (HAS/FJS), a private unit of José Silveira Foundation, a reference hospital for maternal and childcare; and the Maternidade Climério de Oliveira (MCO/UFBA) of the Federal University of Bahia (UFBA) a public institution, considered a reference for high maternal and neonatal risk assistance. Both are located in Salvador, the capital of Bahia state and the largest city of northeast region of Brazil with 2.9 million inhabitants. After consenting, clinical information and oropharyngeal and nasal swab were collected by a well-trained professional from all consecutive mothers at hospital admission. At that time, this procedure was done just as part of this study protocol.
Detection of SARS-CoV-2 was carried out by RT-PCR (GoTaq® Probe 1-Step RT-qPCR System; Promega, USA) following the CDC 2019 Novel Coronavirus (2019-nCoV) Real-Time Reverse Transcriptase (RT)–PCR Diagnostic Panel.7 All women with symptoms for more than 15 days of undetermined etiology also had quantitative IgM/IgG ELISA (COVID-19 IgG/IgM ECO Teste®, ECO Diagnóstica) performed. Newborns whose mothers were positive for SARS-CoV-2 underwent oropharyngeal sample collection for SARS-CoV-2 through RT PCR, between 24 and 48 h of life. Tests were performed at the Virology and Immunology laboratory of the Institute of Health Sciences at UFBA. The mothers with SARS-CoV-2 detected had the placentas examined for pathological abnormalities.
All data were stored and statistical analysis were performed using the statistical package IBM SPSS Statistics for Windows, Version 27.0 (Armonk, NY: IBM Corp). The prevalence of SARS-CoV-2 infection in the mothers and their neonates and the clinical factors associated with SARS-CoV-2 infection were assessed. Differences between the two maternity patients were compared using the Fisher's exact test. The study was approved by the Research Ethics Committee of Maternidade Climério de Oliveira/Universidade Federal da Bahia, in accordance with Resolution 466/12 of the National Health Council and the 2018 Medical Ethics Code of the Federal Council of Medicine.
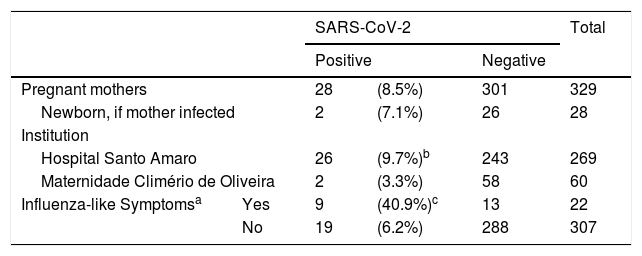
Out of 402 pregnant women giving birth at HAS/FJS and of 101 at MCO/UFBA, 329 (269 and 60, respectively) agreed to participate. 28 (8.5%) had SARS-CoV-2 detected, being 9.7% (26/269) from HAS/FJS and 3.3% (2/60) from MCO/UFBA. 67.8% (19/28) had no symptoms at the time of sample collection. Influenza-like symptoms such as temperature above 38 o C, cough or dyspnea at presentation were 6.6-fold more frequent in those positive for SARS-CoV-2 (Table 1). The most common symptoms were fever (21%), cough (17%), and dyspnea (8%), thorough sample description is shown in Supplementary Tables (S1 – Comparison of samples between institutions, S2 Comparison of clinical presentation between positive and negative pregnant women; and S3 – Comparison of newborns of positive and negative mothers).
SARS-CoV-2 infection in pregnant women screened at admission to Hospital Santo Amaro / Fundação José Silveira or to Maternidade Climério de Oliveira / Universidade Federal da Bahia, June to mid-July, 2020.
Two newborns from the 28 positive mothers had detectable SARS-CoV-2. Both were born at 40 weeks of pregnancy by cesarean section with adequate weight for gestational age. The two mothers whose babies were SARS-CoV-2 positive had neither comorbidities nor experienced any intercurrences during pregnancy. One of these mothers was febrile for two weeks before testing, had a placenta presenting sings of infection, and her newborn had an unveventful hospitalization with normal cranial ultrasonography, normal blood count and negative RT-PCR for SARS-CoV-2 at discharge. The other infected newborn's mother was asymptomatic, also evolved without abnormalities but the placenta had signs of inflammation, villitis and perivilositis. Four more pregnant women had positive anti-coronavirus IgM. Serological evaluation of the newborns showed IgG positive in three of them.
A high prevalence (8.5%) of pregnant women infected with SARS-CoV-2 at hospital admission was observed, and 67.9% of them had no symptoms of coronavirus disease. These rates are lower than those previously reported by Sutton et al. (15.4% and 87.9%, respectively)8 during the peak incidence in New York City/NY, but similar to the 10% prevalence and 74% of asymptomatic pregnant women reported at the University of Birmingham as a WHO Collaborating Center for Women's Health.9 Reports of SARS-CoV-2 asymptomatic infection from case series of universal COVID-19 testing in the obstetric population ranges from 43.5% to 92%.10–13
There was enough evidence in this study of SARS-CoV-2 vertical transmission, as the two newborns found positive were children born to positive mothers with already known infection and delivered by cesarean section with all delivery isolation care. Thus, direct contact either from mother's saliva droplets expelled during speech and/or cough, or caregivers, family members, or even health team members 14,15 could be excluded. Transplacental hematogenic route is being reported, although rarely.16 In our study, the risk could not be assessed as only two newborns were found positive. Not unexpected, both of them had good outcome for their infection, in line with other reports suggesting that COVID-19 in pregnancy does not seem to be a risk factor for disease severity at the neonatal period.10–12,17 In the case of one the two newborns there were inflammatory signs in the placenta, like those reported by Shanes et al.18 who found maternal vascular malperfusion, particularly abnormal or injured maternal vessels, and intervillous thrombi. In general, SARS-CoV-2 infection is uncommon in neonates, with an incidence of 5.6 [95% CI 4.3–7.1] per 10,000 live births in the UK. However, unexpected prevalence of 14.5% has been reported.19,20 Our study has some limitations. On one hand, RT-PCR was only performed for detecting the presence of SARS-CoV-2 as part of this study protocol. On the other hand, only oropharyngeal and nasal swab were collected, when theoretically, umbilical cord blood, amniotic and gastric fluid, anal swabs, or stool from neonates could have been sampled for detecting SARS-CoV-2.21
This report calls attention for a necessity to fast and precise identification of COVID-19 infected pregnant women upon hospital admission for proper implementation of isolation measures to prevent hospital transmission.
FundingThis study was financed by the Fundação José Silveira