Considering that the main route of Mycobacterium leprae transmission is the upper respiratory tract, detection of salivary antibodies can be a useful tool for diagnosing early infection. The study aimed to analyze salivary anti-PGL-1 IgA and IgM antibodies in 169 children aged 4–16 years old, who lived nearby or inside the house of multibacillary or paucibacillary leprosy patients in two endemic cities in Alagoas State – Brazil. Salivary anti-PGL-1 antibodies were quantified by modified ELISA method. The frequency of contact and clinical form of the index case were significantly associated with salivary antibody levels. High frequency of IgM positivity strongly suggests active transmission of M. leprae in these communities. We suggest in the present work that salivary anti-PGL IgA and IgM are important biomarkers to be used for identifying communities with probable active transmission of M. leprae.
Brazil is the second country with the highest incidence of leprosy in the world. In 2015, the country presented a detection rate of 14.06 cases per 100,000 inhabitants.1 Although the number of new cases seems to decrease, it may not represent the reality. For instance, the high incidence of the disease among children means that an active transmission occurs in the community.2 In 2015, the detection rates of leprosy among people under 15 years old in Santana do Ipanema and Rio Largo, two Brazilian cities located in Alagoas State, were 13.77 and 32.81 per 100,000 inhabitants, respectively.3
As the bacteria are not cultivable, secretory antibodies can be a useful tool to detect early infection. The nasopharynx is the main portal of entry for Mycobacterium leprae (M. leprae), and the nasal epithelial cells are an important reservoir of the bacteria.4 As mucosal immune organs and tissues compose an integrated system, saliva is frequently considered to be representative of mucosal humoral immune response. The purpose of the present work was to evaluate salivary anti-phenolic glycolipid 1 antigen (PGL-1) IgA and IgM isotypes among 169 leprosy contacts aged 4–16 years living in the municipalities of Santana do Ipanema and Rio Largo (Alagoas state, Brazil).
MethodsSubjects and sample collectionThe contacts (n=169) included in the study were classified as paucibacillary (PB contacts, n=40) or multibacillary (MB contacts, n=115) contacts, according to clinical form of the index case. Fourteen contacts were not classified because the information was not available in the patients’ medical records. The participants were also classified as household contacts (HH, n=57) or peridomiciliar contacts (PD, n=112). Peridomiciliar contacts were those who were relatives of the index case but did not live in the same house or those who lived close to the index case's house (up to five houses apart). The project was approved by the National Committee for Ethics in Research. Unstimulated saliva samples were collected into tubes, which were transported with ice packs to the laboratory, where they were kept at −20°C until testing (up to three weeks after collection). The presence of lesions and nerve enlargement were investigated at the moment of sample collection. Cases suspected of having the disease were referred to a doctor and excluded from the study.
Detection of salivary anti-PGL-1 antibodiesMicroplates were coated with native PGL-1 at 5mg/L in absolute alcohol for 2h at 37°C (protocol modified from Brito e Cabral et al., 2013).5 After blocking with 1% fetal bovine serum (FBS, LGC Bio, Brazil)-Tris solution for 2h at 37°C, the wells were incubated with previously cenrifuged saliva samples (diluted to 1:50 with 1% FBS-Tris). After 18h at 4°C and washing with 0.05% FBS-Tris solution, anti-human IgA or anti-IgM alkaline phosphatase antibodies (Sigma, USA, 1:1000 in 1% FBS-Tris) were left on the plates for 2h at 37°C. After new incubation for 2h at 37°C, and washing, the substrate solution (1mg/mL p-nitrophenyl phosphate in 10% diethanolamine containing 0.5mM MgCl2, pH 9.8) was added to the wells. After 100min at room temperature, absorbance readings were recorded at 405nm using an ELISA microplate reader. The results were expressed as the OD mean of the values (minus blank). The cut-off was based on the 97th percentile of normal controls.6 Results 30% above the cut-off value were considered to be positive.
Analysis of dataThe data were analyzed using nonparametric tests as the data did not follow a Gaussian distribution (Kolgomorov–Smirnov test). All statistical analysis was performed using GraphPad Prism version 5.0. The level of statistical significance was 5% (p<0.05).
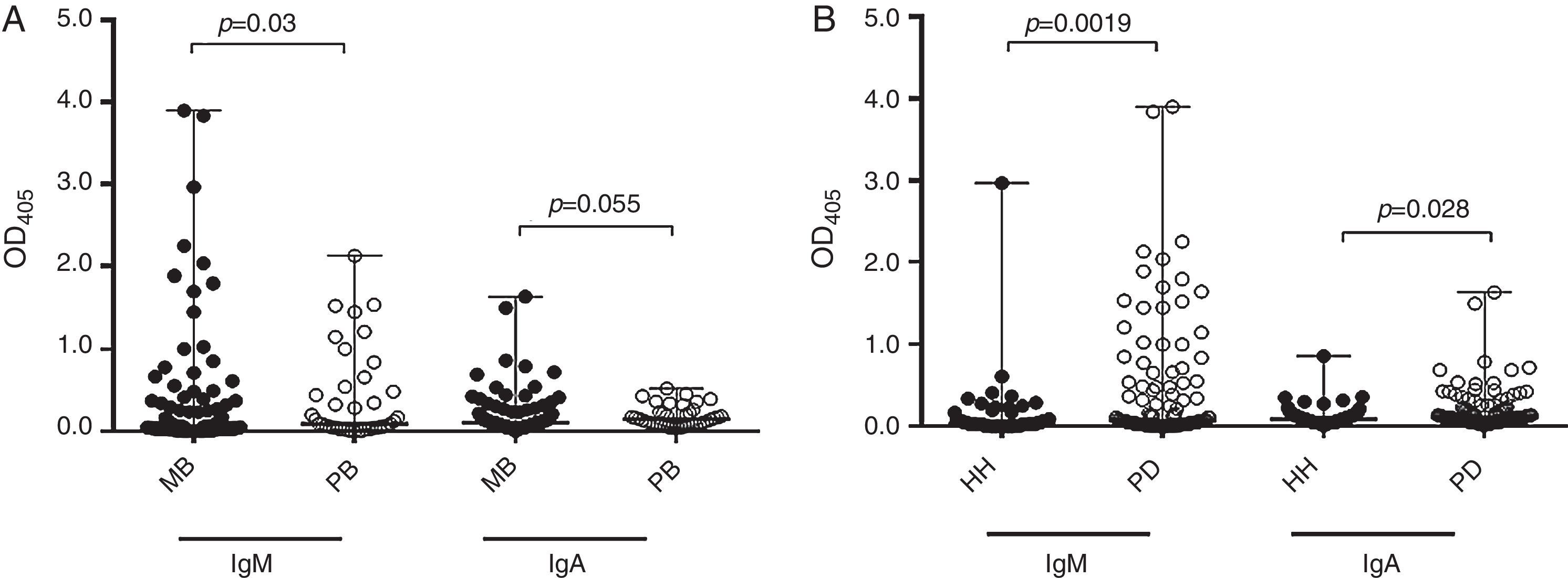
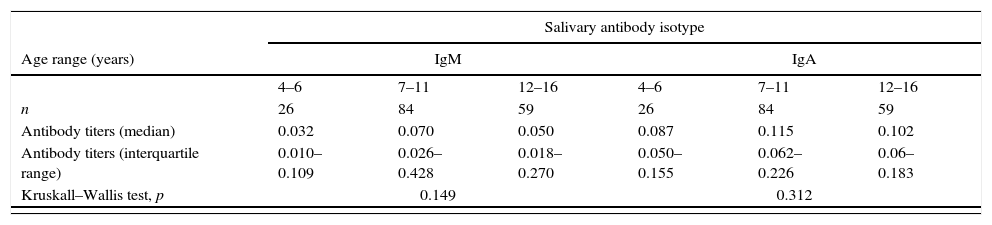
ResultsSalivary anti-PGL-1 IgM presented good correlation to salivary IgA titers (Spearman correlation, r=0.71, p<0.0001). No statistical significance was found regarding the age range, either for IgM or IgA (Kruskall–Wallis test, p=0.149 and p=0.312, respectively, Table 1). No significant differences were either found in IgM or IgA titers in respect to the degree of relationship with the index case (p=0.325 and p=0.590, respectively, Table 1). Contacts who reported having weekly contact with the index case had higher IgM antibody titers than those with daily contact (p=0.04, Table 1). MB leprosy contacts presented higher levels of salivary anti-PGL-1 IgM and IgA (Mann–Whitney test, p=0.03 and p=0.05, respectively) than PB leprosy contacts (Fig. 1A). Interestingly, PD contacts had higher levels of salivary IgM and IgA (Mann–Whitney test, p=0.019 and p=0.028, respectively) than the HH contacts (Fig. 1B).
Titers of salivary anti-PGL1 IgA and IgM in 169 young contacts of leprosy patients according to the age range of leprosy contacts, their degree and frequency of relationship with the index case.
| Salivary antibody isotype | ||||||
|---|---|---|---|---|---|---|
| Age range (years) | IgM | IgA | ||||
| 4–6 | 7–11 | 12–16 | 4–6 | 7–11 | 12–16 | |
| n | 26 | 84 | 59 | 26 | 84 | 59 |
| Antibody titers (median) | 0.032 | 0.070 | 0.050 | 0.087 | 0.115 | 0.102 |
| Antibody titers (interquartile range) | 0.010–0.109 | 0.026–0.428 | 0.018–0.270 | 0.050–0.155 | 0.062–0.226 | 0.06–0.183 |
| Kruskall–Wallis test, p | 0.149 | 0.312 | ||||
| Family or social relationship with the index case | Son/daughter Brother/sister | Grand son/Grand daughter | Nephew/niece/cousin/cousine | Others | Son/daughter Brother/sister | Grand son/Grand daughter | Nephew/niece/cousin/cousine | Others |
|---|---|---|---|---|---|---|---|---|
| n | 32 | 18 | 26 | 93 | 32 | 18 | 26 | 93 |
| Antibody titers (median) | 0.035 | 0.100 | 0.036 | 0.062 | 0.095 | 0.132 | 0.125 | 0.107 |
| Antibody titers (interquartile range) | 0.010–0.088 | 0.023–0.250 | 0.010–0.483 | 0.024–0.324 | 0.050–0.165 | 0.059–0.194 | 0.042–0.263 | 0.067–0.200 |
| Kruskall–Wallis test, p | 0.325 | 0.590 | ||||||
| Frequency of relationship with the index case | Daily | Weeklya | Monthly | N.m. | Daily | Weekly | Monthly | N.m. |
|---|---|---|---|---|---|---|---|---|
| n | 72 | 27 | 5 | 65 | 72 | 27 | 5 | 65 |
| Antibody titers (median) | 0.037 | 0.25 | 0.087 | 0.050 | 0.100 | 0.130 | 0.076 | 0.110 |
| Antibody titers (interquartile range) | 0.010–0.190 | 0.030–0.550 | 0.038–0.399 | 0.02–0.232 | 0.058–0.185 | 0.076–0.260 | 0.062–0.362 | 0.060–0.199 |
| Kruskall–Wallis test, p | 0.04 | 0.499 | ||||||
N.m.=not mentioned.
Levels of salivary anti-PGL-1 antibodies in 169 young contacts of leprosy patients. (A) Median and range of salivary anti-PGL1 IgM and IgA in contacts of multibacillary (MB contacts, n=115) and paucibacillary (PB contacts, n=40) leprosy patients. (B) Median and range of salivary anti-PGL1 IgM and IgA levels in household (HH, n=57) and peridomiciliar (PD, n=112) contacts. Salivary anti-PGL-1 antibodies were detected by modified ELISA method.
With the advent of multidrug therapy the report of new cases of leprosy had a sharp decrease. However, this decline has become less steep in recent years; on the contrary, there has been a rise in leprosy cases including children.6 This makes the goal of eliminating leprosy impossible to be achieved in the next few years,2 remembering that there are possible undiagnosed cases that are hidden sources of bacterial transmission. In addition, there are many unknown aspects regarding the ecology of M. leprae.6 Strategies are necessary to interrupt transmission, such as the development of biomarkers to identify contacts and/or to identify those at risk of developing the disease.7
Mucosal immunity in leprosy is poorly understood, although it is known that the nasal cavity is one of the first sites infected by M. leprae, and the oral cavity can also be affected, as observed in late-diagnosed patients.8 Salivary antibodies of the IgA isotype have been considered as biomarkers of infection, and also of immunity, considering that they may play a role in inhibiting cell adhesion and in opsonophagocytosis.9 Smith et al. (2004), in a follow-up study of people residing in endemic regions for leprosy, found an initial positivity of 1.6% for polymerase-chain reaction of nasal swab and 67.7% for salivary anti-M. leprae IgA.10 A very interesting aspect observed in the study was that the frequency of positivity was higher in certain seasonal periods, especially in the presence of humidity, suggesting that the bacillus remains in the community but not necessarily in the individual.10 In accordance with this hypothesis, Mohanty and colleagues detected viable strains of M. leprae in environmental samples obtained from around the houses of leprosy patients in Ghatampur (India). The prolonged presence of bacilli could play an important role in the continued transmission of leprosy.11
A very low number of studies refer to the presence of anti-PGL1 IgM in saliva,5,9,12,13 which possibly indicates recent infection, since the half-life of IgM-producing plasma cells is only five days, and their levels may be correlated with bacillary load.12 Abe et al. (1984) found a frequency of positivity corresponding to 4.5% (five out of 110 patients).9 We found much higher positivity of salivary anti-PGL1 IgM isotype among leprosy contacts, i.e. 17 out of 47 samples (36.1%) in Rio Largo, and 15 out of 122 samples (12.3%) of children from Santana do Ipanema. Likewise, in a previous study carried out in Crato and Maracanaú cities, state of Ceara, Brazil, 13 out of 135 samples (9.6%) turned out positive for salivary anti-PGL1 IgM.5 In this way, one could infer the magnitude of active transmission in the community. In fact, in 2013, the case detection rate among young people under 15 years old was 13.25 cases per 100,000 individuals in Santana do Ipanema, while no case was detected in Rio Largo. In 2014, no case was detected in the two cities. In 2015, the case detection rate in Santana do Ipanema was 32.81 cases per 100,000 individuals and 13.77 cases per 100,000 individuals in Rio Largo.1
As leprosy infection requires prolonged contact time, those who live in the same house of the index case is believed to be at risk for developing the disease; however, recent reports demonstrated that those who live nearby the index case should also be investigated.14
It is an intriguing fact found in our present work those who lived nearby the index case presented higher levels of salivary antibodies than the household contacts. The paradox tolerance/activation makes the mucosal immune system a challenging task. The mucosal immune response may be affected by various factors, such as soluble or particulate antigens, chemical nature and concentration of antigen, frequency of exposition, gut microbiota composition, environmental antigenic exposure, nutritional status (deficiency of vitamin A), chronic infections with helminths or other parasites.15 PGL-1, for instance, facilitates bacterial adhesion,4 modulates macrophage cytokine and chemokine production, and may lead T cells to anergy.16 In this respect, it is probable that PGL-1 exerts some type of oral tolerance on mucosal immune response.
Natural killer T cells recognize glycolipid antigens presented by CD1d molecule and may also play an important role in oral tolerance by inducing tolerogenic dendritic cells and regulatory T cells, or by deleting antigen-specific T cells.17 These mechanisms could partly explain what may be occurring in children with prolonged and sustained contact with the index case.
Detection of positive salivary IgM among young people suggests that M. leprae transmission is active in the community. For this reason, a strategy at municipal level is extremely urgent in order to reduce the dissemination of the bacillus. Finally, we suggest in the present work that salivary anti-PGL IgA and IgM are important biomarkers to be used for identifying communities with probable active transmission of M. leprae.
Funding informationThis research was financially supported by the MCTI/CNPq/MS-SCTIE [Process 403461/2012-0].
Conflicts of interestThe authors declare no conflicts of interest.
We would like to thank Mrs. Ana Lúcia Carneiro Leal, Mrs. Gilvânia França Vilela, and Mrs. Andrea Márcia Costa de Farias for helpful assistance.