Safety data on the yellow fever vaccine 17DD in People Living with HIV (PLWH) are limited. This study explored the occurrence of post-vaccination 17DD viremia and the kinetics of hematological and liver laboratorial parameters in PLWH and HIV-uninfected participants [HIV(-) controls].
MethodsWe conducted a secondary analysis of a longitudinal interventional trial (NCT03132311) study that enrolled PLWH and HIV(-) controls to receive a single 17DD dose and were followed at 5, 30 and 365 days after vaccination in Rio de Janeiro, Brazil. 17DD viremia (obtained throughreal-time PCR and plaque forming units’ assays), hematological (neutrophils, lymphocytes and platelets counts) and liver enzymes (ALT and AST) results were assessed at baseline and Days 5 and 30 post-vaccination. Logistic regression models explored factors associated with the odds of having positive 17DD viremia. Linear regression models explored variables associated with hematological and liver enzymes results at Day 5.
ResultsA total of 202 PLWH with CD4 ≥ 200 cells/µL and 68 HIV(-) controls were included in the analyses. 17DD viremia was found in 20.0 % of the participants and was twice more frequent in PLWH than in HIV(-) controls (22.8% vs. 11.8 %, p-value < 0.001). Neutrophils, lymphocytes and platelets counts dropped at Day 5 and returned to baseline values at Day 30. 17DD viremia was associated with lower nadir of lymphocytes and platelets at Day 5. ALT levels did not increase post-vaccination and were not associated with 17DD viremia.
Conclusions17DD was safe and well-tolerated in PLWH with CD4 ≥ 200 cells/µL. Post-vaccination viremia was more frequent in PLWH than in controls. Transient and self-limited decreases in lymphocytes and neutrophils occurred early after vaccination. 17DD viremia was associated with lower lymphocytes and platelets nadir after vaccination. We did not observe elevations in ALT after 17DD vaccination.
The Yellow Fever (YF) vaccine (17D or 17DD strains) is widely used, with almost 100 million doses distributed annually worldwide.1 It is one of the most effective vaccines ever made 2 and the key to eliminating YF epidemics.3 Tropical regions of Africa and Central and South America are endemic to YF, and people living in or traveling to those regions should be vaccinated.4 In Brazil, since 2017, in response to a YF outbreak outside the endemic Amazon region, vaccination became recommended nationwide for individuals aged nine months up to 59-years with no contra-indications to the vaccine.5
As a live attenuated viral vaccine, the YF vaccine is contraindicated for individuals with conditions that increase the risk for a serious adverse event, including elders, people with history of thymectomy and people with immunocompromising disorders, such as People Living With HIV (PLWH) with severe immunodeficiency (CD4 < 200 cells/µL or < 15 % in children below 6-years).6
YF vaccine-related serious adverse events are rare and include hypersensitivity reactions, neurological disease (encephalitis, meningitis, autoimmune diseases involving the central and peripheral nervous system, described as YF vaccine Associated Neurologic Disease [YEL-AND]) and viscerotropic disease (generalized multisystem infection, similar to severe forms of the disease, described as YF vaccine Associated Viscerotropic Disease [YEL-AVD]). In Brazil, passive surveillance estimated the incidence of serious adverse events as 0.3 cases per 100,000 administered doses from 2000 to 2015.7
In PLWH, safety data on YF vaccine are limited to observational studies (mostly retrospective) and few recent prospective studies.8-12 Although a case of fatal YEL-AND was reported in a 53-year-old man with undiagnosed HIV infection and a CD4 of 108 cells/µL,13 most studies that included PLWH have reported reassuring results on the safety profile of the YF vaccine in PLWH with CD4 ≥350 cells/µL. The majority of adverse events observed were mild and self-limited, including injection site reactions, nausea, myalgia, fever and laboratorial abnormalities (i.e., neutropenia, lymphopenia, thrombocytopenia, AST and ALT elevations).8,11
The mechanisms leading to YF vaccine serious adverse events remain unclear, with individual host's and vaccine's characteristics probably involved.14 After vaccination, 17D/17DD viruses replicate in the lymphoid and reticuloendothelial tissues.15 This viremia occurs early and is similar to the natural infection's incubation period.16 Some studies found that replication can last longer after vaccination, particularly among elders.17,18 Noteworthy, the causality definition for YEL-AVD requires viral detection in blood or tissues.19
We showed recently that the 17DD vaccine was safe and well tolerated in PLWH.20 However, we found that 17DD viremia was associated with adverse events in PLWH and HIV-uninfected controls (HIV(-) controls).20 In this study, we aimed to further explore factors associated with the occurrence of 17DD viremia in PLWH and HIV(-) controls. In addition, we describe the kinetics of hematological and liver laboratorial parameters and assess whether 17DD viremia and HIV status were associated with the occurrence of laboratorial abnormalities.
MethodsStudy designThis study is a secondary analysis of a prospective cohort study that enrolled PLWH and HIV(-) controls to receive a standard dose of 17DD vaccine at Instituto Nacional de Infectologia Evandro Chagas (INI/Fiocruz) from 2017 to 2018.20 Participants aged 18 and 59 years with no history of YF previous illness or vaccination were eligible for the study. Additional eligibility criteria were not presenting known contraindications to the vaccine, such as immune disorders (i.e., PLWH with CD4 < 200 cells/µL, primary immunodeficiency, malignancy, immunosuppressive or immune response modifying drugs using, thymic dysfunction, having received blood products or immunoglobulins in the last three months), pregnancy or breastfeeding, having received a live attenuated virus vaccine in the last month, current symptoms of severe acute illness or fever ≥ 38 °C. Participants with hypersensitivity to egg, poultry proteins, erythromycin, kanamycin and hereditary fructose intolerance were not enrolled to avoid anaphylactic reactions.
Among PLWH, a recorded CD4 ≥ 200 cells/µL in the past six months and not being on a Chemokine Receptor type 5 (CCR5) antagonist antiretroviral medication (i.e., maraviroc) were required. All women of reproductive age underwent a pregnancy test before vaccine administration. For HIV(-) controls, a non-reactive HIV rapid test was required at enrollment.
All participants received a standard dose of 17DD vaccine produced by Bio-Manguinhos/Fiocruz, administrated subcutaneously.21 At enrollment, medical history and blood samples were obtained. Follow-up visits were scheduled on Day 5, Day 30 and Year 1 after enrollment. Safety laboratory tests (detailed below) were collected at enrollment, Day 5 and Day 30. Yellow fever immunogenicity was measured at enrollment, Day 30 and Year 1 using micro-plaque reduction neutralization - horseradish peroxidase (µPRN, at Laboratory of Virological Technology [LATEV] Fiocruz) and results were expressed as reciprocal of dilution, and titers ≥ 100 were considered reactive.22
For the following analyses, the study population was restricted to participants without serological evidence of prior YF disease/vaccine at enrollment (µPRN < 100) and with available results for 17DD viremia assessment at Day 5 (described below).
Viremia and post-vaccination hematological and liver abnormalities17DD viremia was assessed at Day 0 (pre-vaccination), Day 5 and Day 30 using qualitative real-time Polymerase Chain Reaction (in-house real-time PCR [rt-PCR] performed at the Flavivirus Laboratory of Instituto Oswaldo Cruz [LABFLA] Fiocruz) 23 in serum and urine samples and plaque-forming unit assay (PFU, log10 PFU/mL) in serum samples (performed at LATEV/Fiocruz).24 Participants were classified as having 17DD viremia if any of the assays (rt-PCR serum and urine or PFU) performed at Day 5 resulted positive. All samples collected at Day 0 were negative for 17DD viremia.
Safety laboratory test samples were collected at Day 0 (baseline), at Day 5 and Day 30 visits and included neutrophils, lymphocytes, platelets, Alanine Aminotransferase (ALT) and Aspartate Aminotransferase (AST).
Independent variablesBaseline variables were age at enrollment, sex at birth, Dengue (DENV) and Zika (ZKV) IgG and IgM antibodies (TR DPP ZDC IgM/IgG, Biomanguinhos).25 For PLWH, baseline HIV viral load (RNA, copies/mL, Abbot Park, IL, USA) with a minimum detection limit of 40 copies/mL, CD4 (cells/µL, BD Biosciences, CA, USA), CD4/CD8 ratio, CD4 nadir (lowest CD4 recorded value prior to enrollment), use and duration of antiretroviral therapy were ascertained.
Statistical analysisComparisons between groups were made using the Kruskal-Wallis test for continuous variables and Fisher's exact test or Chi-Square test for categorical variables.
Hematological and liver laboratorial results’ distributions were described using medians and Interquartile Ranges (IQR) as well as by calculating the absolute difference between baseline values and Day 5 values (named delta). For descriptive purposes, the study population was grouped according to HIV status and baseline CD4 counts. Kruskal-Wallis test was used for comparison between Day 0 and Day 5 results within each group.
Logistic regression models were fit to evaluate factors associated with the odds of having 17DD viremia among PLWH and HIV(-) controls. An initial adjusted model included age, sex, DENV IgG, ZKV IgG and a variable that categorized the participants according to HIV status (PLWH vs. HIV(-) controls [reference]). A second model, restricted to PLWH, included age, sex, baseline CD4, CD4/CD8 ratio and HIV-RNA.
Linear regression models were fit to evaluate factors associated with hematological (neutrophils, lymphocytes and platelets counts) and ALT and AST levels at Day 5. Initial models included age, sex, baseline hematological or liver parameters, 17DD viremia and a variable that categorized the participants according to HIV status (PLWH vs. HIV(-) controls [reference]). Secondly, models restricted to PLWH, included age, sex, baseline hematological or liver parameters, 17DD viremia, baseline CD4, CD4/CD8 ratio and HIV-RNA were fit.
Ethical aspectsThis study was approved by INI/Fiocruz Ethics Committee (CAAE: #67136517.9.0000.5262) and registered at Clinicaltrials.gov (NCT03132311). Participants provided written informed consent.
ResultsStudy population characteristicsOut of 300 participants (218 PLWH and 82 HIV(-) controls) that received a standard dose of 17DD vaccine, 12 participants had serological evidence of prior YF disease/vaccine (μPRN titers ≥100) at baseline and 18 had no available 17DD viremia results at Day 5, and therefore were excluded from the analyses. Of the 270 participants included the analyses, 54 participants had evidence of 17DD viremia at Day 5 (20.0 %, 95 % Confidence Interval [95 % CI] 15.5‒25.4) and one participant persisted viremic up to the Day 30 visit (Table 1). When analyzing 17DD viremia positivity by type of sample and detection assay used, we found that 15.8 % of the participants had positive rt-PCR in serum, 5.9 % had quantifiable viable virus measured by PFU and 3 % had positive rt-PCR in urine.
Study population characteristics stratified by evidence of 17DD viremia.
| Negative | Positive | Total | p-value | |
|---|---|---|---|---|
| (n = 216) | (n = 54) | (n = 270) | ||
| Age, years (median, IQR) | 42 (34.4, 50.2) | 43.8 (33, 49.1) | 42 (34, 50.2) | 0.959a |
| 18–29 | 35 (16.2) | 12 (22.2) | 47 (17.4) | 0.400 |
| 30–39 | 61 (28.2) | 10 (18.5) | 71 (26.3) | |
| 40–49 | 64 (29.6) | 19 (35.2) | 83 (30.7) | |
| 50–59 | 56 (25.9) | 13 (24.1) | 69 (25.6) | |
| Sex | 0.565a | |||
| Female | 77 (35.6) | 17 (31.5) | 94 (34.8) | |
| Male | 139 (64.4) | 37 (68.5) | 176 (65.2) | |
| DENV IgM Abb | 0.693a | |||
| Non-reactive, n (%) | 206 (96.3) | 52 (98.1) | 258 (96.6) | |
| Reactive, n (%) | 8 (3.7) | 1 (1.9) | 9 (3.4) | |
| DENV IgG Abb | 0.038a | |||
| Non-reactive, n (%) | 31 (14.5) | 14 (26.4) | 45 (16.9) | |
| Reactive, n (%) | 183 (85.5) | 39 (73.6) | 222 (83.1) | |
| ZKV IgM Abb | 0.487a | |||
| Non-reactive, n (%) | 212 (99.1) | 52 (98.1) | 264 (98.9) | |
| Reactive, n (%) | 2 (0.9) | 1 (2.38) | 3 (1.1) | |
| ZKV IgG Abb | 0.252a | |||
| Non-reactive, n (%) | 127 (59.3) | 36 (67.9) | 163 (61) | |
| Reactive, n (%) | 87 (40.7) | 17 (32.1) | 104 (39) | |
| 17DD serum (rt-PCR) at Day 5 | <0.001a | |||
| Negative | 213 (100) | 11 (20.8) | 224 (84.2) | |
| Positive | 0 (0) | 42 (79.2) | 42 (15.8) | |
| 17DD serum PFU at Day 5 | ||||
| Negative | 216 (100) | 37 (69.8) | 253 (94.1) | <0.001a |
| Positive | 0 (0) | 16 (30.2) | 16 (5.9) | |
| 17DD urine (rt-PCR) at Day 5 | ||||
| Negative | 214 (100) | 45 (84.9) | 259 (97) | <0.001a |
| Positive | 0 (0) | 8 (15.1) | 8 (3) | |
| HIV status | 0.050a | |||
| HIV(-) controls | 60 (27.8) | 8 (14.8) | 68 (25.2) | |
| PLWH | 156 (72.2) | 46 (85.2) | 202 (74.8) | |
| Among PLWH | ||||
| CD4, cells/µL (median, IQR)b | 625 (447, 881) | 664 (517, 1012) | 630 (462, 899) | 0.264a |
| 200‒350 | 13 (8.3) | 4 (8.7) | 17 (8.4) | 0.420 |
| 351‒499 | 38 (24.4) | 7 (15.2) | 45 (22.3) | |
| ≥ 500 | 105 (67.3) | 35 (76.1) | 140 (69.3) | |
| CD4/CD8 ratio (median, IQR)b | 0.8 (0.5, 1.2) | 0.8 (0.6, 1) | 0.8 (0.6, 1.1) | 0.965a |
| < 0.40 | 24 (15.4) | 7 (15.2) | 31 (15.3) | 0.602 |
| 0.40‒0.69 | 38 (24.4) | 9 (19.6) | 47 (23.3) | |
| 0.70‒0.99 | 42 (26.9) | 17 (37) | 59 (29.2) | |
| ≥ 1.00 | 52 (33.3) | 13 (28.3) | 65 (32.2) | |
| HIV-viral load (copies/mL)b | ||||
| < 40 (n,%) | 141 (91.6) | 44 (95.7) | 185 (92.5) | 0.545a |
| ≥ 40 (n,%) | 13 (8.4) | 2 (4.3) | 15 (7.5) |
IQR, Interquartile Range; DENV IgG Ab, Dengue IgG antibody; DENV IgM Ab, Dengue IgM antibody; ZKV IgG Ab, Zika IgG antibody; ZKV IgM Ab, Zika IgM antibody, rt-PCR, Real-time Polymerase Chain Reaction; PFU, Plaque Forming Units’ assay; HIV(-) controls, HIV-uninfected people, PLWH, People Living With HIV.
17DD viremia was twice more frequent in PLWH (22.8 %, 95 % CI 17.3‒29.3) than in HIV(-) controls (11.8 %, 95 % CI 5.6‒22.4, p-value for comparison < 0.001) and was less frequent among participants with DENV IgG antibodies (17.6% vs. 31.1 %, p-value = 0.061). Age, sex and prevalence of ZKV IgG antibodies were not different between participants with positive or negative 17DD viremia. All PLWH were using antiretroviral therapy at baseline, the median CD4 was 630 cells/µL (IQR 462‒899), the median CD4/CD8 ratio was 0.8 (IQR 0.6‒1.11) and 92.5 % had HIV-RNA < 40 copies/mL. These variables were not different between 17DD viremia groups (Table 1).
Compared to HIV(-) controls, PLWH had higher odds of having positive 17DD viremia (adjusted Odds Ratio [Aor = 2.26], p-value = 0.063) (Fig. 1). In a second regression model restricted to PLWH, baseline CD4, CD4/CD8 ratio and HIV-RNA were not associated with the odds of having 17DD viremia.
Factors associated with the occurrence of 17DD viremia in the study population and PLWH. Adjusted logistic regression models are shown that included PLWH and HIV(-) controls (A); and only PLWH (B). Ref, Stratum use as reference; DENV, Dengue Virus; ZKV, Zika Virus; HIV(-) controls, HIV-uninfected people; PLWH, People Living with HIV; CI, Confidence Interval.
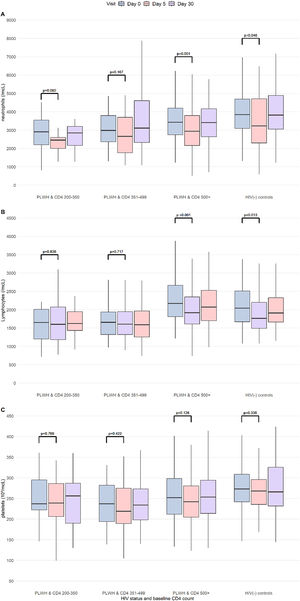
We observed a transient decline of neutrophils and lymphocytes, platelets (minor, non-significant), in PLWH and HIV(-) controls; the nadir levels observed at Day 5 returned to baseline levels at Day 30 (Fig. 2). Decreases in neutrophils and lymphocytes at D5 relative to Day 0 (delta) were greatest in HIV(-) controls and PLWH with CD4 ≥500 cells/µL. However, nadir levels (at Day 5) of neutrophils and lymphocytes were the lowest in PLWH, with CD4 between 200‒350 and 351‒499 cells/µL.
Kinetics of neutrophils, lymphocytes and platelets results (Day 0, Day 5 and Day 30) in the study population. Boxplots showing the density distribution, median in bold, first and third quartiles are shown for neutrophils (A), lymphocytes (B) and platelets (C) by study visit. The study population was categorized according to HIV status and baseline CD4 counts. p-values for comparison between Day 0 and Day 5 results (Kruskal-Wallis). CD4 count is measured cells/µL. PLWH, People Living With HIV, HIV(-) controls: HIV-uninfected controls.
Overall, participants with positive 17DD viremia had greater decrease in lymphocytes (p-value = 0.001) and platelets (p-value = 0.040) at Day 5 than those with negative 17DD viremia. Decreases in neutrophils were similar between the two positive and negative 17DD viremia groups (Suppl. Fig. 1).
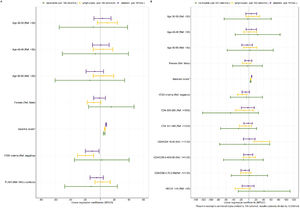
Linear regression models explored factors associated with neutrophils, lymphocytes and platelets counts at Day 5 (Fig. 3). Results at Day 5 were linearly associated with respective baseline values (i.e., neutrophils count at Day 5 and at baseline), while having 17DD viremia was associated with lower lymphocyte and platelets counts at Day 5. HIV status (PLWH vs. HIV(-) controls) was not associated with the levels of neutrophils, lymphocytes and platelets at Day 5. In the models restricted to PLWH, baseline CD4, CD4/CD8 ratio and HIV-RNA were not associated with neutrophils and platelets counts at Day 5. In contrast, low baseline CD4/CD8 ratio (< 0.40) was associated with higher lymphocytes counts at Day 5.
Factors associated with the neutrophils, lymphocytes and platelets at Day 5 in the study population and PLWH. Adjusted linear regression models are shown that included PLWH and HIV(-) controls (A) and only PLWH (B). Ref, Stratum use as reference; HIV(-) controls, HIV-uninfected people; PLWH, People Living With HIV; CI, Confidence Interval.
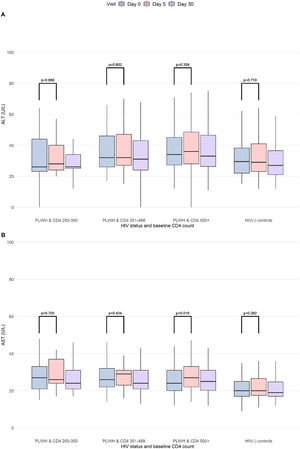
There were no significant elevations in ALT levels post-vaccination (Fig. 4). A non-clinically relevant increase of AST at Day 5 was found among PLWH with CD4 ≥500 cells/µL. ALT and AST levels at Day 5 were also not different in participants with positive or negative 17DD viremia (Suppl. Fig. 2).
Kinetics of ALT and AST results (Day 0, Day 5 and Day 30) in the study population. Boxplots showing the density distribution, median in bold, first and third quartiles are shown for ALT (A) and AST (B) by study visit. The study population was categorized according to HIV status and baseline CD4 counts. p-values for comparison between Day 0 and Day 5 results (Kruskal-Wallis). CD4 count was measured in cells/µL. PLWH, People Living With HIV, HIV(-) controls, HIV-uninfected controls.
Of note, one participant had an ALT of 357 U/L at Day 5 (in combination with AST 195 U/L, total bilirubin 1.6 mg/dL) that decreased to 224 U/L at Day 30 (AST 85 U/L, total bilirubin 0.8 mg/dL). This participant had HIV infection with baseline CD4 levels of 1161 cells/µL and HIV-RNA ≤ 40, obesity (BMI 38 kg/m2) and unknown hepatic steatosis diagnosed after enrollment. 17DD viremia was positive (positive rt-PCR in serum, negative PFU and negative urine) at Day 5 and negative at Day 30. At baseline, liver results were elevated (ALT 222 U/L, AST 122 U/L, total bilirubin 2.09 mg/dL) and the abnormalities found during the study were deemed associated with hepatic steatosis and not with the 17DD vaccine, in accordance with the hepatologist consultant.
In the adjusted linear regression models (Fig. 5), ALT levels at Day 5 were significantly associated with baseline ALT levels but not with 17DD viremia, HIV status, baseline CD4, CD4/CD8 ratio nor HIV-RNA.
Factors associated with ALT and AST levels at Day 5 in the study population and PLWH. Adjusted linear regression models are shown that included PLWH and HIV(-) controls and only PLWH (B). Ref, Stratum use as reference; HIV(-) controls, HIV-uninfected people; PLWH, People Living With HIV; CI, Confidence Interval.
This longitudinal study provides further evidence on YF (17DD) primo-vaccination safety in PLWH with CD4 ≥200 cells/µL. Through an extensive evaluation of post-vaccination 17DD viremia and hematological and liver laboratorial parameters kinetics, we found that PLWH experienced 17DD viremia twice more frequently than HIV(-) controls. Moreover, we observed benign and transient decreases in neutrophils, lymphocytes and platelets at Day 5 after 17DD vaccination. 17DD viremia was independently associated with lower lymphocyte and platelet levels at Day 5. Finally, we did not observe ALT elevations after 17DD vaccination.
We found that 17DD viremia was twice more frequent among PLWH than in HIV(-) controls (23% vs. 12 %). Our results differ from previous prospective studies that have reported a much higher frequency of 17DD/17D viremia (but not different between PLWH and their control groups). In a study from Brazil, 17DD viremia occurred in 40 % of PLWH and 34 % of the controls (defined as at least one detectable result measured by quantitative rt-PCR in plasma samples collected at Days 3, 5, 7 and 14 after vaccination).11 In France, 17D viremia was found in 82 % of PLWH and 77 % of the controls (quantitative rt-PCR in plasma samples collected at Day 7).8 Differences regarding vaccines used (17DD in Brazil and 17D in France), intervals between vaccination and samples collection, and the characteristics of the employed assays may explain the varying 17DD/17D viremia frequencies across the studies. Moreover, the high baseline prevalence of DENV IgG antibodies (83 %) found in our study may provide additional insights. Heterologous immunity, characterized by cross-reactivity and cross-protection from other flaviviruses’ previous infections (i.e., DENV, Japanese encephalitis virus and ZKV), may result in lower 17DD viremia.26,27 In a 17DD dose-response study from Brazil, 17DD viremia (measured by PFU) was less frequent in participants with DENV antibodies.28 Similarly, in our study 17DD viremia was less frequent among participants with DENV IgG antibodies (18% vs. 31 %). Nonetheless, the association between DENV IgG antibodies and 17DD viremia was non-significant in the adjusted regression models. Importantly, though we have found that HIV status was associated with the occurrence of 17DD viremia, there was no clear association between specific HIV biomarkers (i.e., baseline CD4, CD4/CD8 ratio and HIV-RNA) and the odds of 17DD viremia. These negative findings may be due to a lower number of participants with low CD4, low CD4/CD8 ratio and high HIV-RNA included.
We observed significant decreases in neutrophils at Day 5 after 17DD vaccination. Baseline neutrophil levels were the major factor associated with Day 5 levels although a non-significant linear association of lower baseline CD4 and lower neutrophil at Day 5 was observed. Previous studies suggested that neutrophil levels fall up to 10 days after vaccination and recover one month after vaccination, suggesting that neutrophil nadir values observed at Day 5 could be even lower if measured at Day 10.29-31 In PLWH, in which lower levels of neutrophils were not uncommon,32 neutropenia could be aggravated following 17DD vaccination.
The kinetics of lymphocytes observed in our study was similar between PLWH and HIV(-) controls. 17DD viremia was independently associated with lower lymphocyte counts at Day 5, which could be potentially explained by a more enhanced acute immune response among 17DD viremic participants, leading to homing of peripheric lymphocytes to lymphoid tissues.33,34 Early and transient drop of lymphoid cells, natural killer cells, and lymphocytes T and B, has been described following 17D/17DD vaccination in healthy adults.34,35 In a subanalysis of our main study, we further described a significant drop in lymphocytes T CD4+ at Day 5, in both PLWH and controls, returning to baseline levels at Day 30.36 Decrease of CD4+ T lymphocytes and relative increase of CD8+ T lymphocytes had been documented following 17D/17DD vaccination.8,35,37
Minor decreases in platelet counts were observed at Day 5 and were associated with lower baseline platelet levels and with the occurrence of 17DD viremia. Flaviviruses infections often result in a decrease in the platelet count as result of direct viruses toxicity, immunological platelet destruction, megakaryocytes (cells responsible for platelets production in the bone marrow) destruction and impaired megakaryopoiesis.38 Early and transient drop in platelet levels following 17D/17DD vaccine has been reported in healthy adults and PLWH.8,11 Finally, minimal liver laboratorial abnormalities were found in this study. ALT levels did not increase post-vaccination and were not associated with 17DD viremia or HIV infection status.
Our results should be interpreted with caution. This study included mostly PLWH with high baseline CD4 and undetectable HIV-RNA, underpowering the analyses to detect significant effects of advanced HIV infection on 17DD viremia and laboratorial abnormalities following 17DD vaccination. Nevertheless, our study has strengths that should be highlighted. To our knowledge, this study has included and prospectively followed the largest sample of PLWH exposed to 17DD primo vaccination, providing critical data on YF vaccine safety in PLWH and a detailed panel of safety laboratorial kinetics. Moreover, to avoid potential confounding of non-primo vaccination (since they are less likely to present with post-vaccination adverse events), we have restricted the study population to participants without serological evidence of prior YF vaccination/disease. Finally, we have also explored the potential effect of heterologous immunity from previous flaviviruses infections (i.e., DENV and ZKV antibodies) on 17DD viremia.
ConclusionsThe YF vaccine 17DD was safe in PLWH with CD4 ≥200 cells/µL. Post-vaccination 17DD viremia was twice higher in PLWH than in HIV(-) controls. Transient and self-limited decreases in lymphocytes, neutrophils and platelets were observed. 17DD viremia occurrence was associated with lower lymphocytes and platelets nadir after vaccination. YF vaccination of individuals oblivious of their HIV infection should not pose additional risk (assuming those with CD4 < 200 cells/µL would be less likely to have undisclosed HIV infection). Altogether, the results from this study support the current recommendation and the safety of YF vaccine in PLWH with CD4 ≥200 cells/µL.
FundingThis work was funded by National Council for Scientific and Technological Development – CNPq and Presidência da Fundação Oswaldo Cruz/ Vice-presidência de Pesquisa e Coleções Biológicas VPPCB/Fiocruz – Chamada CNPq/Fiocruz n 16/2017– PROEP/PEC (# 420674/2017-9). This work was also supported by Instituto Nacional de Infectologia Evandro Chagas (INI/Fiocruz) and Coordenação de Vigilância em Saúde e Laboratórios de Referência(CVSLR)/FIOCRUZ/ MS. B.G. acknowledges funding from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ).