Antiretroviral therapy increased the survival and life expectancy of People living With HIV (PWH). Frailty-related syndromes among older PWH (aged 50+ years) may affect their Health-related Quality of Life (HQoL). Additionally, the COVID-19 pandemic has impacted health-related outcomes. This study aimed to estimate the prevalence of frailty and pre-frailty among older PWH, and to explore associations of HQoL with the study assessment period and frailty status.
MethodsCross-sectional study conducted pre- (23-Mar-2019 to 5-Mar-2020) and post-COVID-19 pandemic onset (23-Jun-2021 to 5-May-2022), among older PWH at INI-Fiocruz, the largest cohort of PWH in Rio de Janeiro, Brazil. We measured frailty using Fried assessment, consisting of five domains: unintentional weight loss; self-reported exhaustion, weakness, slow walking speed, low physical activity. HQoL was assessed using the ACTG SF-21, which contains 21 questions divided into 8 domains. We used Chi-Square test, Fisher's exact test, Kruskal-Wallis and ranksum test for comparisons.
ResultsWe included 250 older PWH: 109 (43.6 %) pre- and 141 (56.4 %) post-COVID-19 pandemic onset. Median age was 60-years (IQR: 55‒64). Most self-identified as cisgender men 152 (60.8 %), Pardo/Black 146 (58.4 %), with completed secondary education or less 181 (72.7 %) and low income 132 (52.8 %). Overall, prevalence of frailty and pre-frailty were 9.2 % (95 % CI: 8.1‒10.3) and 61.6 % (95 % CI: 54.0‒69.2). Prevalence of frailty in the pre- and pos-COVID-19 pandemic periods were 7.3 % and 10.6 % (p = 0.66). HQoL scores were lower among participants with frailty compared to those with non-frailty and pre-frailty in all eight domains, and among those included in the post-COVID-19 compared to pre-COVID-19 period for four domains.
ConclusionsWe observed low prevalence of frailty, but high prevalence of pre-frailty among older PWH. Frailty status did not differ according to the COVID-19 assessment period. Assessment of frailty and HQoL should be incorporated in clinical practice for older PWH. Programs to reverse or prevent frailty should be implemented within the public health system.
In 2021, 38.4 million people were living with HIV and 28.7 million were accessing Antiretroviral Therapy (ART) globally.1 Due to the development of more effective and safe antiretroviral drugs as well as ART scale-up, the number of AIDS-related deaths decreased by 52% from 2010 to 2021.1 In Brazil, 960,000 people were living with HIV in 2021, with 700,000 on ART.2 The AIDS detection rate decreased from 22.5 to 16.5 cases per 100,000 inhabitants between 2011 and 2021.2
Although life expectancy has significantly improved for People living With HIV (PWH), disparities remain based on region, education, income level, and access to healthcare.3 Among 30,688 PWH from the Caribbean, Central and South America network for HIV epidemiology (CCASAnet), overall life expectancy increased from 31.0 (95% CI 29.3‒32.8) to 69.5 years (95% CI 67.2‒71.8) from 2003‒2008 to 2013‒2017.4 As a consequence, the older PWH population (defined as those aged 50-years or older) and aging-related health problems are both increasing. Prevalence of multiple comorbid conditions (multimorbidity) among older PWH is considerably higher compared to people of similar age not living with HIV in different cohorts, including in Brazil.5
The development of chronic and acute age-related diseases and physiological decline contribute to frailty.6 Frailty can be defined as a cumulative physiological decline over time, which impacts homeostatic regulation and health status.7 The concept of frailty involves physical, cognitive, social, emotional, and economic aspects.8 As other geriatric-related syndromes, frailty should be screened and assessed during PWH routine clinical care to prevent or reverse frailty process. Frailty-related syndromes among older PWH may impact their quality of life.9
Quality of life comprises several aspects, specifically functional capacity, socioeconomic level, social interaction, intellectual activity, family support and health status.10 Health-related Quality of Life (HQoL) is a dimension of quality of life that reflects the impact of disease and treatment on a person's ability to perform daily activities and affects biological, cognitive, and mental health aspects of wellbeing.
In Brazil, the COVID-19 pandemic has disproportionately impacted sexual and gender minorities, and socioeconomically marginalized populations, impacting health-related outcomes.11,12 We have previously reported that lower educational level and older age were associated with lower HQoL at Instituto Nacional de Infectologia Evandro Chagas, Fundação Oswaldo Cruz (INI-Fiocruz), the largest cohort of PWH in Rio de Janeiro, Brazil.13 Moreover, the COVID-19 pandemic disrupted access to the HIV care continuum for PWH in this cohort, such as adequate care visits, viral load monitoring, and optimal medication possession ratio.14
In this study, we aimed to estimate the prevalence of frailty and pre-frailty among older PWH followed at INI-Fiocruz cohort, and to compare characteristics of this population according to the study assessment period (pre- or post-COVID-19 pandemic onset) and frailty status. Furthermore, we explored associations of HQoL with the study assessment period and frailty status.
Material and methodsStudy designThis was a cross-sectional study nested at the INI-Fiocruz cohort conducted in two periods to reflect pre- (March 23, 2019 to March 5, 2020) and post-COVID-19 pandemic onset (June 23, 2021 to May 5, 2022). INI-Fiocruz is a reference institution for care, research, and training in infectious diseases in Rio de Janeiro, Brazil, established in 1918. The INI-Fiocruz cohort is a longitudinal observational clinical cohort with PWH receiving HIV care since 1986, as described elsewhere.13-16 For this study, inclusion criteria were: (1) Age 50-years or older at the day of assessment, (2) Confirmed HIV infection, (3) Currently using antiretroviral therapy. Exclusion criteria were: (1) Participants with Parkinson disease; (2) MINI-Mental State Examination (MMSE) score <18 points; (3) Any clinical condition that impaired participation and compliance with study procedures.
The INI-Fiocruz Institutional Review Board (IRB) reviewed and approved this study on January 23, 2018 (CAAE #76609317400005262). All participants signed written informed consent before participation. All participants received reimbursement for transportation and lunch.
ProceduresPotential participants were contacted by telephone and invited to participate in the study. Upon arriving at the clinic, a study investigator offered informed consent and assessed inclusion and exclusion criteria. Then, the investigator administered a questionnaire containing questions concerning gender, sociodemographic characteristics, frailty assessment, HQoL, history of falls, medication use/adherence, and substance use.
Frailty assessmentFrailty was measured using a modified version of Fried's assessment, which contains five domains: (1) Unintentional weight loss; (2) Self-reported exhaustion, (3) Weakness (grip strength), (4) Slow walking speed, (5) Low physical activity.17,18 This method has been previously used to assess frailty among PWH.19-21 Frailty is defined as participant meeting three or more domains criteria; pre-frailty as two or one criteria; not fragile if no criteria.17
Unintentional weight loss was defined as loss of ≥ 4.5 Kg without intention or diet. Exhaustion was assessed using two items of the Center for Epidemiological Depression Scale (CES-D), validated among Brazilians aged ≥ 60-years.22 We asked how often in the past week: (1) “I felt everything I did was an effort.”; (2) “I could not get going.” Possible answers were in a 4-Likert scale (0-rarely or none of the time [<1-day], 1-some of the time [1‒2 days], 2-moderately or much of the time [3‒4 days], 3-almost all the time [5‒7 days]). Participants answering “2” or “3” to either of these questions were categorized as frail by the exhaustion criterion. We used the SAEHAN® hydraulic dynamometer to assess the grip strength (Kgf) three consecutive times. We defined frailty for weakness criterium according to gender and Body Mass Index (BMI).17 Slow walking speed was evaluated through the Gait Speed, where participants were instructed to walk four meters twice. Frailty for this criterium was defined according to gender and height.17 Low physical activity was evaluated using the short version of the International Physical Activity Questionnaire (IPAQ) which assesses the time and effort of practicing physical activities performed in the last 7-days.23 Frailty for this criterium was calculated according to MET (Metabolic Equivalent)-minutes week.
Health-related quality of life assessmentHQoL was assessed using a modified version of the SF-21 questionnaire (ACTG SF-21), previously used among PWH initiating first- and second-line ART and under virological failure.24-28 The ACTG SF-21 contains 21 questions divided into 8 domains: general health perception, physical functioning, role functioning, social functioning, cognitive functioning, pain, mental health, and energy/fatigue. A standardized score ranging from 0 (worst HQoL) to 100 (best HQoL) was calculated for each domain.24-28
CovariablesSociodemographic variables were: age (median [Interquartile Range: IQR], stratified in 50‒59, 60‒69 and ≥ 70 years), gender (cisgender man, cisgender woman, transgender woman), race (White, Pardo and Black), schooling (primary or less, secondary and superior), income (family monthly income stratified in low: ≤ 2 minimum wages; middle: > 2 to 6 minimum wages; high: > 6 minimum wages; 1 minimum wage = BRL1212 or USD230), residence (Rio de Janeiro city or metropolitan area) and housing (living alone or not). BMI was stratified into: (1) underweight (< 18.5 kg/m2); (2) adequate weight (18.5- < 25 kg/m2); (3) overweight (25- < 30 kg/m2); (4) obese (≥ 30 kg/m2). MINI-Mental State Examination (MMSE) scores range from 0 to 30 points and presence of cognitive problems (yes/no) was defined according to score and schooling.29 We collected any substance use (marijuana, cocaine, crack, club drugs [e.g., ecstasy, MD], hallucinogens [e.g., LSD, mushrooms, ketamine] and others), and binge drinking (5 or more doses of alcohol in an interval of a few hours [2‒4 h]) in the past 30 days, and tobacco use (never, former or current). ART was stratified in Dolutegravir (DTG)-based regimen, Efavirenz (EFV)-based regimen and other. Adequate ART adherence was considered when a participant reported no missing dose in the past 30 days. The history of falls was evaluated with the question: “Did you have any unintentional fall in the last 6 months?”. Results were stratified into yes (unintentional fall in the last 6 months) and no.
Statistical analysisData were collected in a standard form and entered a RedCap database. The prevalence of frailty and pre-frailty were presented as percentages, with 95 % Confidence Interval (95 % CI). We compared characteristics of older PWH according to the assessment period (pre- or post-COVID-19 pandemic onset) and frailty status (non-frailty, pre-frailty, and frailty). Lastly, we compared median HQoL scores and IQR of older PWH according to the assessment period (pre- or post-COVID-19 pandemic onset) and frailty. We used Chi-Square test, Fisher's exact test, Kruskal-Wallis and Ranksum test for comparisons.
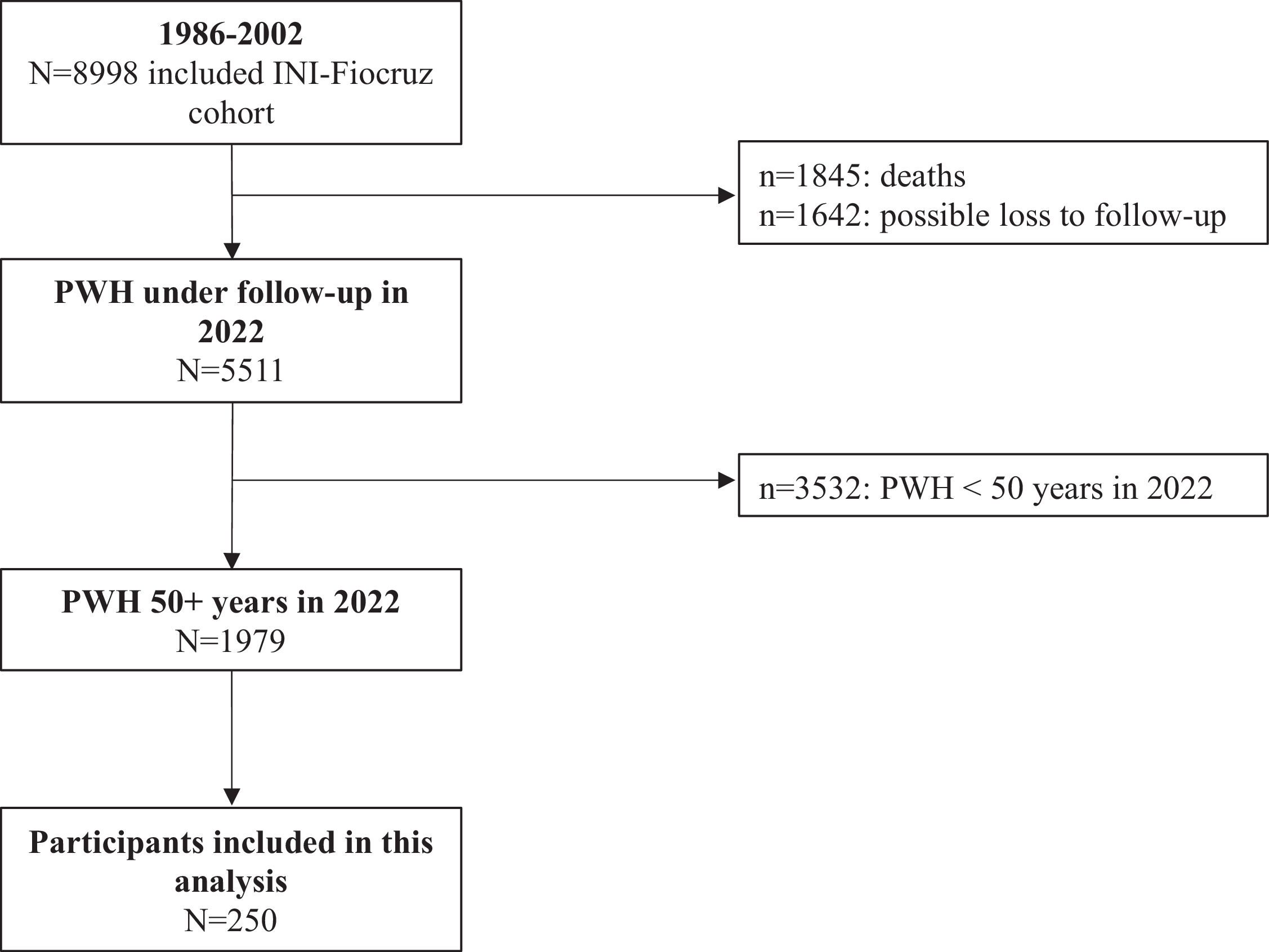
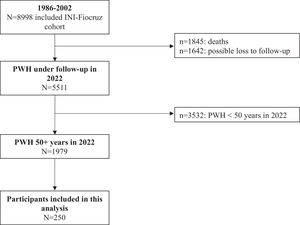
ResultsFrom 1986 to 2022, 8998 PWH were included in the INI-Fiocruz cohort (Fig. 1). Of these, 5511 PWH were under follow-up and using ART in 2022; 35.9 % (n = 1979/5511) aged ≥ 50 years. Between March 2019 and May 2022, 252 individuals were evaluated and 250 were included in this study: 109 (43.6 %) pre- and 141 (56.4 %) post-COVID-19 pandemic onset. Two individuals were excluded due to MMSE score < 18 points.
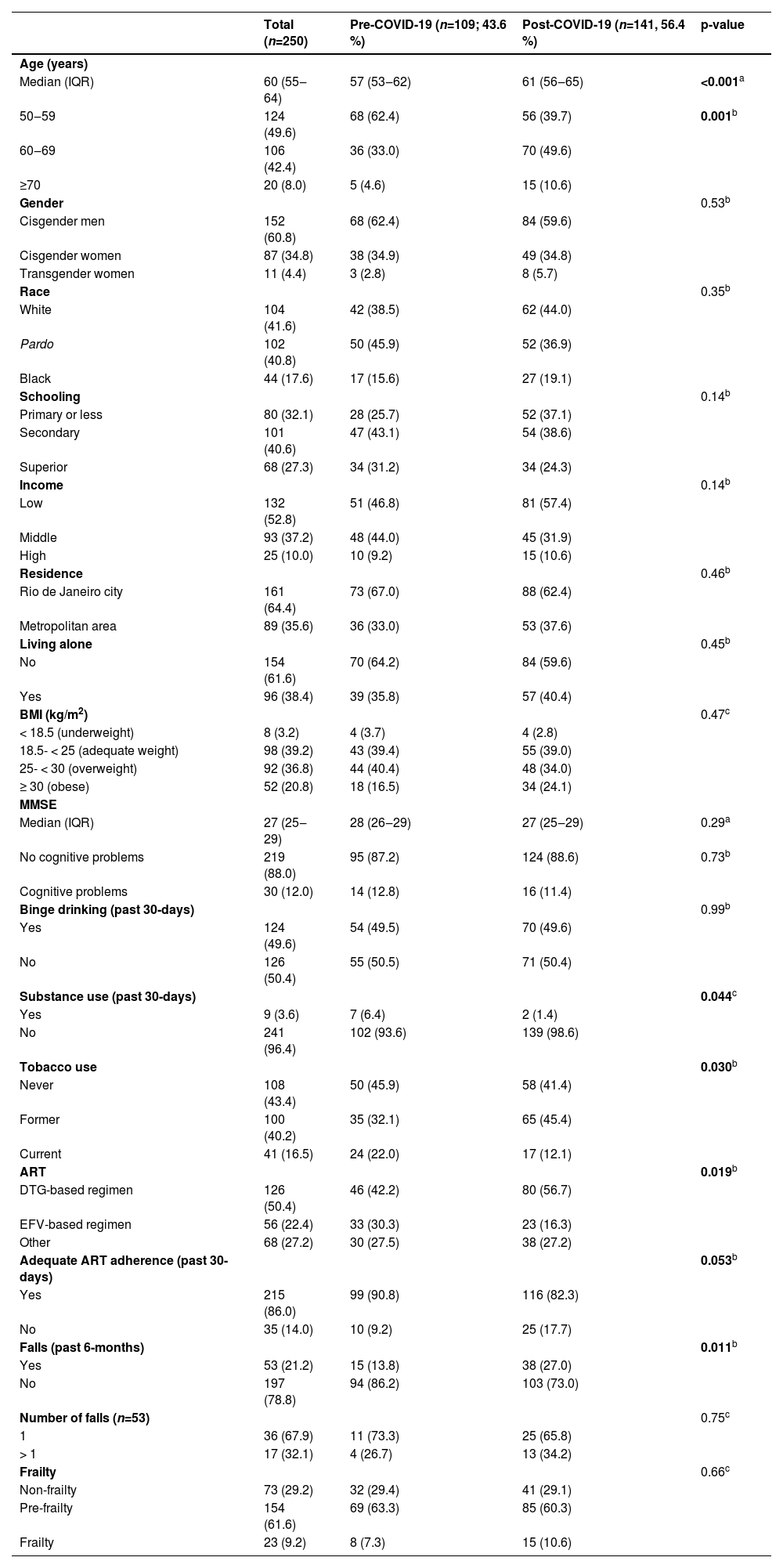
The median age among participants was 60 years (IQR: 55‒64), with 124 (49.6 %) aged 50‒59 years, 106 (42.4 %) 60‒69 years and 20 (8.0 %) ≥ 70 years (Table 1). Most participants self-identified as cisgender men 152 (60.8 %), while 11 (4.4 %) identified as transgender women. Most were Pardo or Black (n=146; 58.4 %), completed secondary education or less (n=181; 72.7 %), had low income (n=132; 52.8 %), with residence in Rio de Janeiro city (n=161; 64.4 %) and lived with someone (n=154; 61.6 %). Only 8 (3.2 %) participants were underweighting and 52 (20.8 %) were obese. Thirty (12.0 %) participants had cognitive problems according to MMSE score, 124 (49.6 %) reported binge drinking, 9 (3.6 %) any substance use, and 141 (56.7 %) ever smoked. Almost half (n=126; 50.4 %) were in DTG-based regimen and 215 (86.0 %) reported adequate ART adherence. A total of 53 (21.2 %) participants reported unintentional falls in the past 6 months; among them, 17 (32.1 %) reported more than one fall.
Characteristics of older PWH according to assessment period (pre- or post-COVID-19 pandemic onset). Rio de Janeiro, Brazil (2019‒2022).
| Total (n=250) | Pre-COVID-19 (n=109; 43.6 %) | Post-COVID-19 (n=141, 56.4 %) | p-value | |
|---|---|---|---|---|
| Age (years) | ||||
| Median (IQR) | 60 (55‒64) | 57 (53‒62) | 61 (56‒65) | <0.001a |
| 50‒59 | 124 (49.6) | 68 (62.4) | 56 (39.7) | 0.001b |
| 60‒69 | 106 (42.4) | 36 (33.0) | 70 (49.6) | |
| ≥70 | 20 (8.0) | 5 (4.6) | 15 (10.6) | |
| Gender | 0.53b | |||
| Cisgender men | 152 (60.8) | 68 (62.4) | 84 (59.6) | |
| Cisgender women | 87 (34.8) | 38 (34.9) | 49 (34.8) | |
| Transgender women | 11 (4.4) | 3 (2.8) | 8 (5.7) | |
| Race | 0.35b | |||
| White | 104 (41.6) | 42 (38.5) | 62 (44.0) | |
| Pardo | 102 (40.8) | 50 (45.9) | 52 (36.9) | |
| Black | 44 (17.6) | 17 (15.6) | 27 (19.1) | |
| Schooling | 0.14b | |||
| Primary or less | 80 (32.1) | 28 (25.7) | 52 (37.1) | |
| Secondary | 101 (40.6) | 47 (43.1) | 54 (38.6) | |
| Superior | 68 (27.3) | 34 (31.2) | 34 (24.3) | |
| Income | 0.14b | |||
| Low | 132 (52.8) | 51 (46.8) | 81 (57.4) | |
| Middle | 93 (37.2) | 48 (44.0) | 45 (31.9) | |
| High | 25 (10.0) | 10 (9.2) | 15 (10.6) | |
| Residence | 0.46b | |||
| Rio de Janeiro city | 161 (64.4) | 73 (67.0) | 88 (62.4) | |
| Metropolitan area | 89 (35.6) | 36 (33.0) | 53 (37.6) | |
| Living alone | 0.45b | |||
| No | 154 (61.6) | 70 (64.2) | 84 (59.6) | |
| Yes | 96 (38.4) | 39 (35.8) | 57 (40.4) | |
| BMI (kg/m2) | 0.47c | |||
| < 18.5 (underweight) | 8 (3.2) | 4 (3.7) | 4 (2.8) | |
| 18.5- < 25 (adequate weight) | 98 (39.2) | 43 (39.4) | 55 (39.0) | |
| 25- < 30 (overweight) | 92 (36.8) | 44 (40.4) | 48 (34.0) | |
| ≥ 30 (obese) | 52 (20.8) | 18 (16.5) | 34 (24.1) | |
| MMSE | ||||
| Median (IQR) | 27 (25‒29) | 28 (26‒29) | 27 (25‒29) | 0.29a |
| No cognitive problems | 219 (88.0) | 95 (87.2) | 124 (88.6) | 0.73b |
| Cognitive problems | 30 (12.0) | 14 (12.8) | 16 (11.4) | |
| Binge drinking (past 30-days) | 0.99b | |||
| Yes | 124 (49.6) | 54 (49.5) | 70 (49.6) | |
| No | 126 (50.4) | 55 (50.5) | 71 (50.4) | |
| Substance use (past 30-days) | 0.044c | |||
| Yes | 9 (3.6) | 7 (6.4) | 2 (1.4) | |
| No | 241 (96.4) | 102 (93.6) | 139 (98.6) | |
| Tobacco use | 0.030b | |||
| Never | 108 (43.4) | 50 (45.9) | 58 (41.4) | |
| Former | 100 (40.2) | 35 (32.1) | 65 (45.4) | |
| Current | 41 (16.5) | 24 (22.0) | 17 (12.1) | |
| ART | 0.019b | |||
| DTG-based regimen | 126 (50.4) | 46 (42.2) | 80 (56.7) | |
| EFV-based regimen | 56 (22.4) | 33 (30.3) | 23 (16.3) | |
| Other | 68 (27.2) | 30 (27.5) | 38 (27.2) | |
| Adequate ART adherence (past 30-days) | 0.053b | |||
| Yes | 215 (86.0) | 99 (90.8) | 116 (82.3) | |
| No | 35 (14.0) | 10 (9.2) | 25 (17.7) | |
| Falls (past 6-months) | 0.011b | |||
| Yes | 53 (21.2) | 15 (13.8) | 38 (27.0) | |
| No | 197 (78.8) | 94 (86.2) | 103 (73.0) | |
| Number of falls (n=53) | 0.75c | |||
| 1 | 36 (67.9) | 11 (73.3) | 25 (65.8) | |
| > 1 | 17 (32.1) | 4 (26.7) | 13 (34.2) | |
| Frailty | 0.66c | |||
| Non-frailty | 73 (29.2) | 32 (29.4) | 41 (29.1) | |
| Pre-frailty | 154 (61.6) | 69 (63.3) | 85 (60.3) | |
| Frailty | 23 (9.2) | 8 (7.3) | 15 (10.6) |
IQR, Interquartile Range; BMI, Body Mass Index; ART, Antiretroviral Therapy; DTG, Dolutegravir; EFV, Efavirenz; Bold, p < 0.05.
Compared to participants included pre-COVID-19 onset, those included post-COVID-19 pandemic were older (median age 61 years [IQR: 56‒65] vs. 57 years [IQR: 53‒62]; p < 0.001) and reported less substance use (1.4% vs. 6.4 %; p = 0.044). More participants were former smokers (45.4% vs. 32.1 %; p = 0.030), were on DTG-based regimen (56.7% vs. 42.2 %; p = 0.019), reported inadequate ART adherence (17.7% vs. 9.2 %; p = 0.053) and unintentional falls (27.0% vs. 13.8 %; p = 0.011).
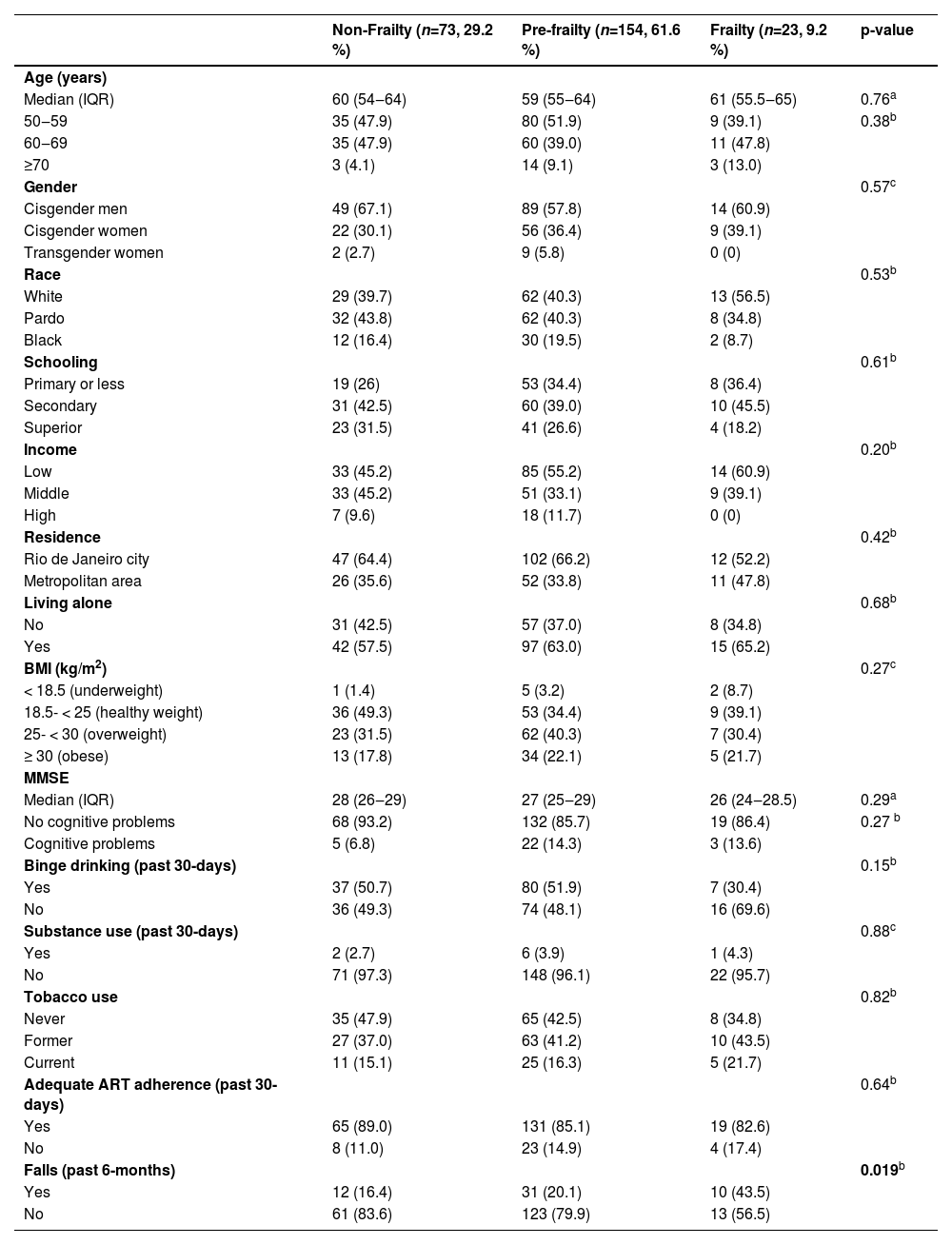
Prevalence of frailty and pre-frailty were 9.2 % (95 % CI: 8.1‒10.3) and 61.6 % (95 % CI: 54.0‒69.2), respectively. Frailty status did not differ according to the COVID-19 inclusion period. We observed no association of evaluated variables with frailty, except for unintentional fall: 43.5 % for frailty, 20.1 % pre-frailty, and 16.4 % non-frailty (p = 0.019) (Table 2).
Characteristics of older PWH according to frailty status. Rio de Janeiro, Brazil (2019‒2022).
| Non-Frailty (n=73, 29.2 %) | Pre-frailty (n=154, 61.6 %) | Frailty (n=23, 9.2 %) | p-value | |
|---|---|---|---|---|
| Age (years) | ||||
| Median (IQR) | 60 (54‒64) | 59 (55‒64) | 61 (55.5‒65) | 0.76a |
| 50‒59 | 35 (47.9) | 80 (51.9) | 9 (39.1) | 0.38b |
| 60‒69 | 35 (47.9) | 60 (39.0) | 11 (47.8) | |
| ≥70 | 3 (4.1) | 14 (9.1) | 3 (13.0) | |
| Gender | 0.57c | |||
| Cisgender men | 49 (67.1) | 89 (57.8) | 14 (60.9) | |
| Cisgender women | 22 (30.1) | 56 (36.4) | 9 (39.1) | |
| Transgender women | 2 (2.7) | 9 (5.8) | 0 (0) | |
| Race | 0.53b | |||
| White | 29 (39.7) | 62 (40.3) | 13 (56.5) | |
| Pardo | 32 (43.8) | 62 (40.3) | 8 (34.8) | |
| Black | 12 (16.4) | 30 (19.5) | 2 (8.7) | |
| Schooling | 0.61b | |||
| Primary or less | 19 (26) | 53 (34.4) | 8 (36.4) | |
| Secondary | 31 (42.5) | 60 (39.0) | 10 (45.5) | |
| Superior | 23 (31.5) | 41 (26.6) | 4 (18.2) | |
| Income | 0.20b | |||
| Low | 33 (45.2) | 85 (55.2) | 14 (60.9) | |
| Middle | 33 (45.2) | 51 (33.1) | 9 (39.1) | |
| High | 7 (9.6) | 18 (11.7) | 0 (0) | |
| Residence | 0.42b | |||
| Rio de Janeiro city | 47 (64.4) | 102 (66.2) | 12 (52.2) | |
| Metropolitan area | 26 (35.6) | 52 (33.8) | 11 (47.8) | |
| Living alone | 0.68b | |||
| No | 31 (42.5) | 57 (37.0) | 8 (34.8) | |
| Yes | 42 (57.5) | 97 (63.0) | 15 (65.2) | |
| BMI (kg/m2) | 0.27c | |||
| < 18.5 (underweight) | 1 (1.4) | 5 (3.2) | 2 (8.7) | |
| 18.5- < 25 (healthy weight) | 36 (49.3) | 53 (34.4) | 9 (39.1) | |
| 25- < 30 (overweight) | 23 (31.5) | 62 (40.3) | 7 (30.4) | |
| ≥ 30 (obese) | 13 (17.8) | 34 (22.1) | 5 (21.7) | |
| MMSE | ||||
| Median (IQR) | 28 (26‒29) | 27 (25‒29) | 26 (24‒28.5) | 0.29a |
| No cognitive problems | 68 (93.2) | 132 (85.7) | 19 (86.4) | 0.27 b |
| Cognitive problems | 5 (6.8) | 22 (14.3) | 3 (13.6) | |
| Binge drinking (past 30-days) | 0.15b | |||
| Yes | 37 (50.7) | 80 (51.9) | 7 (30.4) | |
| No | 36 (49.3) | 74 (48.1) | 16 (69.6) | |
| Substance use (past 30-days) | 0.88c | |||
| Yes | 2 (2.7) | 6 (3.9) | 1 (4.3) | |
| No | 71 (97.3) | 148 (96.1) | 22 (95.7) | |
| Tobacco use | 0.82b | |||
| Never | 35 (47.9) | 65 (42.5) | 8 (34.8) | |
| Former | 27 (37.0) | 63 (41.2) | 10 (43.5) | |
| Current | 11 (15.1) | 25 (16.3) | 5 (21.7) | |
| Adequate ART adherence (past 30-days) | 0.64b | |||
| Yes | 65 (89.0) | 131 (85.1) | 19 (82.6) | |
| No | 8 (11.0) | 23 (14.9) | 4 (17.4) | |
| Falls (past 6-months) | 0.019b | |||
| Yes | 12 (16.4) | 31 (20.1) | 10 (43.5) | |
| No | 61 (83.6) | 123 (79.9) | 13 (56.5) |
Bold, p < 0.05.
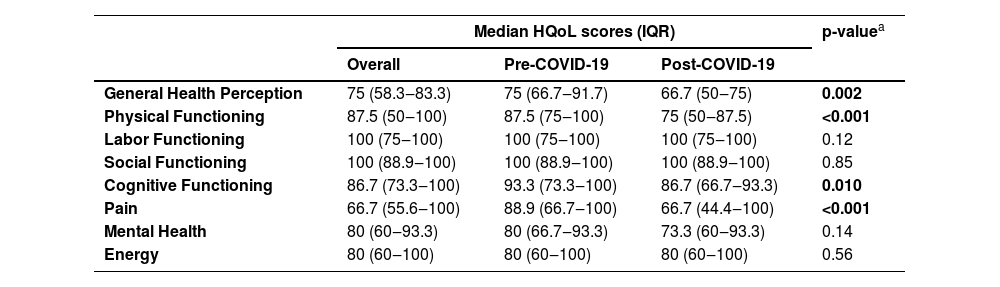
Overall, median HQoL scores varied from 66.7 for pain domain to 100 for labor functioning and social functioning (Table 3). Compared to participants included pre-COVID-19, those included post-COVID-19 had lower scores for general health perception (p = 0.002), physical functioning (p < 0.001), cognitive functioning (p = 0.010) and pain (p < 0.001).
Median health-related quality of life scores (IQR) among older PWH according to assessment period (pre- or post-COVID-19 pandemic onset), Rio de Janeiro, Brazil (2019‒2022).
| Median HQoL scores (IQR) | p-valuea | |||
|---|---|---|---|---|
| Overall | Pre-COVID-19 | Post-COVID-19 | ||
| General Health Perception | 75 (58.3‒83.3) | 75 (66.7‒91.7) | 66.7 (50‒75) | 0.002 |
| Physical Functioning | 87.5 (50‒100) | 87.5 (75‒100) | 75 (50‒87.5) | <0.001 |
| Labor Functioning | 100 (75‒100) | 100 (75‒100) | 100 (75‒100) | 0.12 |
| Social Functioning | 100 (88.9‒100) | 100 (88.9‒100) | 100 (88.9‒100) | 0.85 |
| Cognitive Functioning | 86.7 (73.3‒100) | 93.3 (73.3‒100) | 86.7 (66.7‒93.3) | 0.010 |
| Pain | 66.7 (55.6‒100) | 88.9 (66.7‒100) | 66.7 (44.4‒100) | <0.001 |
| Mental Health | 80 (60‒93.3) | 80 (66.7‒93.3) | 73.3 (60‒93.3) | 0.14 |
| Energy | 80 (60‒100) | 80 (60‒100) | 80 (60‒100) | 0.56 |
HQoL, Health-related Quality of Life; IQR, Interquartil Range; HQoL scores range from 0 to 100.
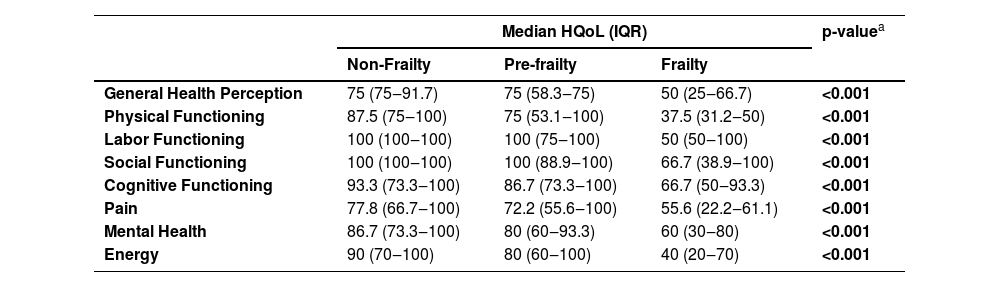
Participants with frailty had lower HQoL scores compared to those with pre-frailty and non-frailty in all eight domains (p < 0.001) (Table 4). For participants with frailty, median HQoL scores varied from 37.5 (physical functioning) to 66.7 (social functioning and cognitive functioning). For participants with non-frailty, median HQoL scores varied from 75 (general health perception) to 100 (labor functioning and social functioning).
Median health-related quality of life scores (IQR) among older PWH according to frailty status. Rio de Janeiro, Brazil (2019‒2022).
| Median HQoL (IQR) | p-valuea | |||
|---|---|---|---|---|
| Non-Frailty | Pre-frailty | Frailty | ||
| General Health Perception | 75 (75‒91.7) | 75 (58.3‒75) | 50 (25‒66.7) | <0.001 |
| Physical Functioning | 87.5 (75‒100) | 75 (53.1‒100) | 37.5 (31.2‒50) | <0.001 |
| Labor Functioning | 100 (100‒100) | 100 (75‒100) | 50 (50‒100) | <0.001 |
| Social Functioning | 100 (100‒100) | 100 (88.9‒100) | 66.7 (38.9‒100) | <0.001 |
| Cognitive Functioning | 93.3 (73.3‒100) | 86.7 (73.3‒100) | 66.7 (50‒93.3) | <0.001 |
| Pain | 77.8 (66.7‒100) | 72.2 (55.6‒100) | 55.6 (22.2‒61.1) | <0.001 |
| Mental Health | 86.7 (73.3‒100) | 80 (60‒93.3) | 60 (30‒80) | <0.001 |
| Energy | 90 (70‒100) | 80 (60‒100) | 40 (20‒70) | <0.001 |
HQoL, Health-related Quality of Life; IQR, Interquartil Range; HQoL scores range from 0 to 100.
In this study, we estimated the prevalence of frailty and pre-frailty among older PWH in a large cohort from Rio de Janeiro, Brazil. HQoL was lower among older PWH with frailty compared to those with non-frailty and pre-frailty. HQoL was also lower among older PWH included in post-COVID-19 compared to pre-COVID-19 pandemic period for four out of eight HQoL domains. Our study increases the body of knowledge about aging and HIV and reinforces the importance of including frailty and HQoL during clinical assessment of older PWH.
The prevalence of frailty among older PWH in our study was similar to that estimated among 8556 older Brazilians included in the ELSI-Brasil study (9.0 %; 95 % CI: 8.0‒10.1)19 which also used Fried methodology to assess frailty.17 This result suggests similarities in the aging process of PWH being followed in our cohort compared to the overall population. Importantly, PWH followed at INI-Fiocruz have access to multidisciplinary care, including cardiology, neurology, physiotherapist, nutritionist, and social services. Previous studies among older PWH conducted in Brazil, France, Mexico and United States estimated frailty ranging from 2.9 % to 19.4 %.20,21,30,31 However, comparisons with other studies should take into consideration differences in methodologies to assess frailty, aging strata, and local settings. Frailty is potentially reversible if treated promptly when detected.32 Prevention of frailty among older PWH is paramount, especially among those with pre-frailty, which represented more than 60 % of our study population. Therefore, efforts to reverse or prevent frailty should be encouraged by health professionals. Public health systems should incorporate programs to promote lifestyle modifications encompassing more physical activities in daily routines including regular exercise and nutritional education among the aging population. Food insecurity, also shown to be associated with frailty among PWH33 and poor adherence to ART,34 should be taken into account by public health policy makers as well.
Our study confirmed the association between worse HQoL and frailty among PWH, as reported previously.20,35,36 The ACTG SF-21 instrument used measured eight HQoL domains.27 Other studies have also shown associations of frailty with the domains of HQoL evaluated here, including neurocognitive impairment association with increased risk of frailty in an observational cohort evaluating aging and HIV, the ACTG A5322 study.37 In a study conducted in Uganda, major depressive disorder was associated with decreasing mean grip strength,38 one of the domains used by Fried to estimate frailty. In another study conducted in France, pain was associated with frailty and pre-frailty.39
We observed worse HQoL among older PWH included post-COVID-19 compared to the pre-COVID-19 pandemic period in four QoL domains: general health perception, physical functioning, cognitive functioning, and pain. Although data were not collected longitudinally, some hypotheses can be inferred. First, individuals included in the post-pandemic period were older than those included pre-pandemic (median 61 [IQR: 56‒65] vs. 57 [IQR: 53‒62]), which may explain lower physical functioning and cognitive functioning. The COVID-19 pandemic led to an increase in psychological distress and negative emotions such as pessimism, which could have impacted participants’ perception of health and pain. These results underscore the importance of HQoL screening among older PWH, especially after the COVID-19 pandemic onset.
This study has limitations. We included a convenience sample of older PWH followed in an HIV cohort. However, our sample size (n = 237) was based on 2019 data showing that 35.5 % (n = 1615/4547) of PWH under follow-up at INI-Fiocruz cohort were 50 years or older and estimated the overall prevalence of frailty among older PWH as 19 %, as reported in a study conducted in Salvador, Brazil.40 Due to the cross-sectional design, causality and directionality could not be inferred. Most variables were self-reported, thus potentially subject to social desirability and recall biases.
ConclusionsLow prevalence of frailty and high prevalence of pre-frailty were observed among older PWH in our cohort. Frailty status did not differ according to the COVID-19 inclusion period. HQoL was lower among older PWH with frailty compared to those with non-frailty and pre-frailty, and among participants included post-COVID-19 compared to those pre-COVID-19. Assessment of frailty and HQoL should be incorporated in clinical practice for older PWH. Programs to reverse or prevent frailty should be implemented within the public health system.
Author's contributionBG, VGV and SWC are responsible for the INI-Fiocruz Cohort of People Living with HIV. TST, RE and SWC conceived and designed this study. TST, LG, LDF, RE and SWC designed the case report forms. DA, JSJ and FAM collected study data and performed frailty assessments. FL supervised data entry. TST did the statistical analyses and drafted this manuscript. SWC supervised the analysis and manuscript preparation. DA, JSJ, FAM, LG, LDF, RE, VGV, BG and SWC revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Availability of data and materialsA complete deidentified dataset sufficient to reproduce the primary study findings will be made available upon request to the corresponding author, following approval of a concept sheet summarizing the analyses to be done.