The standard of care therapy of chronic hepatitis C with the combination of pegylated interferon and ribavirin for 24 or 48 weeks was a remarkable accomplishment of the past decade. However, sustained virological responses rates of about 80% (genotypes 2–3) and 50% (genotype 1) were not satisfactory especially for patients infected with genotype 1. Important advances in the biology of HCV have made possible the development of the direct-acting antiviral agents boceprevir and telaprevir with substantial increase in the rates of sustained virological response with shorter duration of therapy for a large number of patients. However, the complexity of triple therapy is higher and several new side effects are expected suggesting greater expertise in the patient management. Anemia and disgeusia are frequent with boceprevir while cutaneous rash, ranging from mild to severe, is expected with telaprevir. Higher risk of drug–drug interactions demand further clinical consideration of the previous well-known adverse events of pegylated interferon and ribavirin. Identification and prompt management of these potential new problems with boceprevir and telaprevir are crucial in clinical practice for optimizing treatment and assuring safety outcomes to HCV-genotype 1 patients.
The standard of care (SOC) therapy of chronic hepatitis C with the combination of pegylated interferon (PegIFN) and ribavirin (RBV) for 24 or 48 weeks was a remarkable therapeutic accomplishment of the past era. However, sustained virological responses (SVR) rates of about 80% (genotypes 2–3) and 50% (genotype 1) were not satisfactory especially for patients infected with genotype 1.1 Important advances in the knowledge of the structure of HCV proteins, in conjunction with the development of a subgenomic replicon system and a cell culture model that enables productive HCV infection,2,3 have made possible the development of new direct-acting antiviral agents (DAAs) with substantial increase of SVR rates with shorter duration of therapy for a large number of patients.4 Recently, new treatments with the protease inhibitors (PIs) boceprevir (BOC) and telaprevir (TVR) have brought additional chances of cure. However, the complexity of triple therapy is higher and several new side effects and risk of drug–drug interactions are anticipated. Identification and appropriate management of these potential problems are crucial in clinical practice for optimizing treatment and assuring safety outcomes to HCV patients. Therefore, the purpose of this paper is to provide an overview of clinical data regarding safety aspects of PIs including adverse events (AEs) and drug-to-drug interactions.
Proteases as potential targets of DAAsEach step of HCV life cycle offers a potential target for DAA therapy.5 The NS2 and NS3/4A are viral peptidases involved in the post-translational processing of HCV proteins. NS3 is a multifunctional viral protein containing a serine protease domain in its N-terminal third (approximately 180 amino acids) and a helicase NTPase domain in its C-terminal two-third, and NS4A is a cofactor of its proteinase activity. NS3 must assemble with its cofactor NS4A to catalyze HCV polyprotein cleavage.
Preclinical data has demonstrated experimentally that HCV containing defective NS3/4A activity could not replicate.6 This concept was the basis for the recent development of two first-generation NS3/4A anti-protease agents – TVR (Incivek®; Vertex Pharmaceuticals, Cambridge, MA, USA).7 and BOC (Victrelis®; Merck, Kenilworth, NY, USA),8 both recently approved to treat chronic hepatitis in Europe, USA and in Brazil. When used in combination with PegIFN and RBV, these PIs improve substantially the SVR rates in both treatment-naïve patients and in those with previous virological failure. Nevertheless, the addition of these new agents increased the complexity of HCV treatment, as new AEs and several drug–drug interactions, leading to the discontinuation of all antiviral treatments in 9–20% of cases.9–13 In addition, resistance mutations are a new main concern as they are more likely to develop with ongoing exposure to PIs. Thus, updated guidelines of HCV treatment have set up strict stopping rules based on inadequate virological responses or severe side-effects.1,14
TVR is bioavailable and absorbed in the small intestine.15 For optimal exposure, TVR must be taken with food rich in fat. Systemic exposure (area under the curve – AUC) is increased by 237% with a standard fat meal (533kcal and 21g fat) compared with the fasted state.16 Clinical trials have also demonstrated that BOC AUC increased up to 65% when taken with food, but type of meal and timing are not essential.17
Adverse events with DAAsThe triple therapy with BOC or TVR has distinctive AEs. The most important side-effects associated with BOC are anemia, neutropenia and dysgeusia (altered sense of taste). With TVR, the AEs are slightly different from BOC as skin rash and anorectal symptoms (discomfort and pruritus) are more frequent. Dose reductions of IPs should not be used in the management of AEs, as suboptimal doses of these drugs will promote the emergence of resistant HCV species resulting in treatment failure.
Anemia with PI treatmentAnemia, defined as hemoglobin levels below 10g/dL, is a recognized RBV related event with considerable increased rate by the addition of TVR or BOC to the SOC HCV treatment. In clinical trials, triple therapy resulted in a 20–26% increase in the rate of anemia with PIs compared to SOC.14 The frequency of anemia is higher for triple therapy with BOC as compared to TVR (about 50% and 40%, respectively). It seems to result from bone-marrow suppressive effect and not haemolysis.18
The impact of anemia on the SVR rate was distinct for the two drugs in clinical trials. A recent pooled retrospective analysis of two phase 3 studies (ADVANCE and ILLUMINATE) evaluated efficacy outcomes of TVR in relation to the occurrence of anemia in 1239 patients.19 The frequency of anemia was 41% and 26% in patients with triple therapy with TVR and SOC, respectively. SVR rates in patients with anemia were 74% and 50% with triple therapy and SOC, respectively. Conversely, SVR rates of patients without anemia were 73% and 41% with triple therapy and SOC treatment. These data strongly suggested that anemia had no effect on SVR rates in treatment naïve patients. Although anemia was more frequent in patients who received a TVR-based regimen as compared to patients on SOC treatment, the hemoglobin values gradually improved after the end of TVR at week 12 and were similar to those on SOC by week 20.19 In addition, patients with TVR-based therapy and SOC who developed anemia had 72% and 58% of RBV dose reduction due to AEs, compared to 11% and 6% of patients without anemia. SVR was achieved by 76% and 54% of patients with RBV dose reduction in the TVR-based therapy and SOC, respectively, compared with 72% and 41% of patients without RBV dose reduction in TVR-based therapy and SOC, respectively. This data suggested that management of treatment-related anemia with dose reduction of RBV did not impact SVR with TVR-based therapy.19
The relationship of anemia in BOC-based regimen with SVR was also investigated in a retrospective analysis of 1097 treatment-naïve and 403 previous-treatment failure patients included in BOC trials. Anemia occurred in 49% and in 29.7% of patients treated with BOC regimen and SOC, respectively. The management of anemia consisted of EPO use by 78.5% of anemic patients on BOC regimens and 29% of those treated with SOC. In contrast to that observed in TVR trials, the SVR rate was higher in patients who developed anemia compared to patients without anemia in both naïve or experienced patients.19 However, since about 80% of anemic patients took EPO in BOC trials, the relationship of SVR and reduction of RBV, anemia and EPO use has not been completely established with TVR regimens so far.
According to the recent UK guidelines for the use of the PIs in HCV treatment14 management of anemia (Hb<10g/dL) and significant neutropenia (absolute neutrophil count<750/mm3) should be conducted as follows: (a) RBV dose should be started at full treatment dose and dose reduction instituted for anemia at decrements of 200g; (b) reduction in dose of IFN, if bone marrow suppression is evident; (c) EPO administration may be considered and used until Hb>12g/dL; supportive treatment with blood transfusion should be considered in extreme circumstances and significant neutropenia should be managed according to current practice for SOC treatment. In addition, consideration should be given to dose reduction of PegIFN. Importantly, the dose of PI should not be reduced for managing neutropenia or bone marrow suppression. If required due to the severity of neutropenia, the PI should be stopped completely.
Dermatological events during PI treatmentThe typical dermatological reactions with SOC consist of generalized pruritus and skin xerosis, with eczematiform lesions accentuated by erythematous papules and microvesicles that are often excoriated, with predominance on the extremities and truncal skin sites exposed to friction.20
Use of DAAs results in additional skin disorders. Higher frequency and severity of dermatological reactions have been reported with TVR21–23 and infrequently with BOC,24,25 as part of triple therapy regimens. This fact is especially important in clinical practice as extra patient management considerations are required by HCV-treating physicians.
A pooled analysis of the dermatological safety profile among 1346 patients who received at least one dose of TVR and 764 patients who received at least one dose of placebo in five placebo-controlled phase II/III trials of TVR,9,10,12,22 rash and pruritus were observed in 55% and 51% of patients treated with TVR-based regimen compared to 33% and 26% with placebo.
In TVR trials, rash events were graded by severity into three grades21,22 (Table 1). More than 90% of rash events related to TVR were Grade 1 or 2 (mild/moderate) and did not progress. The extent of rash in TVR clinical trials grades 1, 2, and 3 were 37%, 14% and 5%, respectively. In 92% of patients with rash, no progression to a more severe grade was observed. Approximately 6% of all patients required discontinuation of TVR as a result of skin lesions that resolved with discontinuation of the drug. About 50% of all rash events started during the first four weeks of TVR use, with the remaining 50% starting until week 12. The median time to onset of rash of any grade was 25 days (ranging from 1 to 350 days), suggesting that it can occur at any time during treatment.26 After stopping TVR at week 12, the incidence of rash was comparable between TVR and placebo-treated patients.27
Grading and management of Telaprevir rash severity.a
| Grade | Description | Management |
|---|---|---|
| Grade 1 – mild | Localized skin eruption and/or limited skin eruption with or without associated pruritus | Telaprevir interruption generally is not necessary |
| Grade 2 – moderate | Diffuse skin eruption involving up to 50% of body surface area with or without superficial skin peeling, pruritus, or mucous membrane involvement with no ulceration | Telaprevir interruption generally is not necessary• For progressive eruption telaprevir should be discontinued first.• Consider interrupting ribavirin and/or peginterferon if no improvement in eruption within 7 days of stopping telaprevir, or earlier if rash worsens. |
| Grade 3 – severe | Generalized rash involving EITHER>50% of body surface area OR rash presenting with any of the following characteristics:• Vesicles or bullae• Superficial ulceration of mucous membranes• Epidermal detachment• Typical or atypical target lesions• Palpable purpura/non-blanching erythema | Telaprevir must be stopped immediately• Interrupt ribavirin and/or peginterferon if no improvements in rash with 7 days of stopping telaprevir, or earlier if rash worsens. |
| Life-threatening or systemic reactions | Stevens–Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS), rash that requires therapy with systemic corticosteroids | Permanent discontinuation of all treatment |
Based on a systematic retrospective assessment review of photographs and biopsies of all rash events reported in Phase III TVR trials, the expert panel of dermatologists concluded that the visual appearance and histopathology of rash associated with TVR are comparable to the rash associated with SOC, though TVR-associated rashes were of increased severity and extent, which may occur any time during treatment, and resolve over weeks after discontinuation of TVR. In addition, the biopsies were not suggestive of vasculitis.26
There is no worldwide consensus on the definition of severe cutaneous adverse reaction (SCAR); however, the consensus panel defined that dermatological conditions that are life-threatening and frequently attributed to drug therapy were reported as SCAR, including Stevens–Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS), and acute generalized exanthematous pustulosis (AGEP). Clinical investigators in the TVR development program reported six subjects with SCAR and the panel assessed four as suspected SCAR (one definitive SJS, one definitive DRESS and two possible DRESS cases). Nine out of 11 suspected DRESS cases did not have systemic organ involvement while organ involvement was unconfirmed in two (FDA accessed 12 March, 2012). SJS and TEN are very acute events, with a mortality rate of 25% during hospitalization. DRESS is more progressive and less severe, with mortality rate around 10%.28
The mechanism of TVR-related rash remains unknown and no predictors have been identified. An analysis of multiple HLA alleles performed to determine if the genetic background may increase or decrease the risk of developing a rash during TVR-based regimen did not reveal a strong association of any HLA allele with rash.26
The second dermatological AEs associated with TVR was pruritus, in general associated with rash but could also be seen without it.
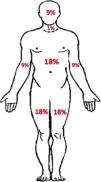
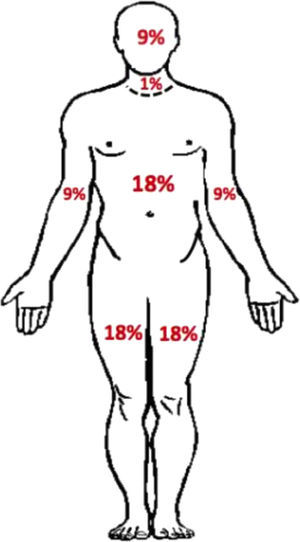
Treatment and management of rashThe recommendations for grading and monitoring the dermatological reactions and for discontinuation of TVR, PegIFN and RBV have been based on the extension and features of dermatological reactions. An estimate of body surface area (BSA) has been used as an indicator of the severity of a dermatological reaction (Fig. 1).
One important point to HCV-treating physicians is to be able to distinguish between usual dermatitis and SCAR. The most frequent TVR-dermatitis is a single entity that generally begins during the first four weeks of therapy, but can occur at any time during treatment. The reaction is an eczematous dermatitis similar to that observed with SOC, but is more frequent and severe with TVR-regimen. Typical rash include pruritus and skin dryness and is stable. The continuation of the triple therapy is possible in Grades 1 and 2 or even in Grade 3 with appropriate management. However, in the rare SCAR presentation, potentially life-threatening if unrecognized or unmanaged, immediate treatment discontinuation is mandatory.27
According to some authors27 some cases of Grade 3 dermatitis reaction affecting more than 50% of body surface but with no signs of SJS, TEN, DRESS, EM or AGEP, may be manageable using topical corticosteroids without treatment discontinuation. In such cases, hospitalization is required to close follow up and intervention in case of signs of progression. Experienced dermatologists should be responsible for patient management. The maintenance of Peg/RBV was allowed in Phase III studies of TVR after the cessation of this drug, to keep the chance of SVR while minimizing the risk of DRESS or SJS. However, the less common but potentially life-threatening reactions such as SJS, TEN and DRESS required prompt cessation of all drugs.27 Differential diagnosis based on biological signs and symptoms has been suggested27 to help physicians distinguishing between TVR-related dermatitis, where antiviral treatment can be continued and supportive treatment given, and the less common but potentially harmful SJS and DRESS reactions (Table 2).
Biological signs and symptoms to distinguish among the most severe telaprevir-related cutaneous adverse reactions (SCARS): drug reaction with eosinophilia and systemic symptoms (DRESS), Stevens–Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN).26
| DRESS | SJS or TEN |
|---|---|
| Onset from 5–10 weeks after drug use | Rapidly progressive exanthema |
| Rapidly progressive exanthema in >50% of BSAa | Skin pain |
| Presence of bullae and vesicles | Mucosal involvement at more than two sites |
| Prolonged fever in general >38.5°C | Blisters or epidermal detachment |
| Facial edema | Atypical target lesions |
| Enlarged lymph nodes | |
| Eosinophilia >10% | |
| Atypical lymphocytes | |
| Rises in ALT, AP (≥2 times upper normal limit) | |
| Rise in creatinine (≥150% basal level) |
In the case of rash grade 1 or 2, patients can benefit from skin care that may limit symptoms and allow antiviral therapy to be continued for as long as possible optimizing the likelihood for viral clearance with TVR-based therapy. Good skin care practices include prophylactic application of emollient creams and lipid-rich lotions, rather than aqueous lotions or ointments after shower or bath, when the skin is still hydrated.
It is important to keep in mind that TVR discontinuation in any case is definitive. Hence, the major aim is not to underestimate any skin reactions, but also to avoid stopping treatment if unnecessary. The final decision might take in account the clinical evaluation of a dermatologist.
Gastrointestinal disorders with BOC and TVRDysgeusia, usually a metallic taste, has an increased frequency in patients receiving BOC and PegIFN/RBV (35% in SPRINT-1 and -2; 44% in RESPOND-2) compared with patients receiving SOC alone (16% in SPRINT-1 and -2; 11% in RESPOND-2).11,13,25 AEs such as dry mouth, nausea, vomiting and diarrhea were observed with BOC regimen, but diarrhea and vomiting were more common in patients with TVR regimen compared to SOC.
Anorectal symptoms occurred more frequently in TVR (26.2%) compared with control arms (5.4%) in TVR trials.10,12,22 The symptoms included hemorrhoids, anal pruritus/discomfort or rectal burning usually within the first two weeks of TVR therapy. Most events were mild/moderate and rarely lead to discontinuation of HCV treatment. All symptoms were resolved after interruption of TVR.
The mechanisms to explain anorectal symptoms are unknown and no association with dermatological AEs has been evident. Patients should undergo anal examination pre-treatment in order to exclude previous local lesions. Symptomatic and non-specific care may be considered for managing anorectal disorders, including topical corticosteroids and systemic antihistamine in case of pruritus.
Drug interactions with BOC and TVRThe adequate concentration of DAAs is critical to HCV treatment success. Drug interactions with potential to decrease DAAs levels can result in lower efficacy and development of drug resistance.18,19,30 In contrast, drug–drug interactions that increase DAAs levels and exposure increase also the risk of AEs. Hence, the effective management of drug–drug interactions is essential to optimize the benefits of treatment of patients infected with HCV genotype 1.18 Therefore, physicians should explore carefully the history of drugs use prescribed or not and also plant-based agents. Physicians are also advised to consult the available databases regarding potential drug–drug interactions.
The knowledge of the main mechanisms of drug interactions contributes to assess the risk and to implement appropriate actions in order to avoid their occurrence.
Pharmacokinetic interactions involving changes in metabolism are the most important drug interactions. The CYP enzymes are responsible for the most part of drug metabolism. As a consequence, the most frequent and important mechanism for drug interactions is CYP inhibition, with potential to promote high levels of drugs and related toxicity.29,30 To date, most drug interactions with new DAAs originate from in vitro studies, case reports and clinical suspicion. Some interactions are theoretical.
About 60% of medications are metabolized by CYP3A, thus several drug interactions must be considered with BOC and TVR as they are both substrates and inhibitors of CYP3A29,31 (Table 3).
Drug interactions with boceprevir (BOC) and telaprevir (TVR).
| Co-administered drug | Pharmacokinetic parameters | Comments/recommendations | |||||
|---|---|---|---|---|---|---|---|
| Boceprevir | Telaprevir | Co-administered drug | |||||
| AUC | Cmax | AUC | Cmax | AUC | Cmax | ||
| Antiretroviral drugs | |||||||
| Efavirez | ↓ 19% | ↓ 8% | ↓ 26% | ↓ 9% | ↑ 20% (BOC) | ↑ 11% (BOC) | TVR and BOC: combination should be avoided; this may result in loss of therapeutic effect. |
| Tenofovir | ↑ 8% | ↑ 5% | – | – | ↑ 5% (BOC)↑ 30% (TVR) | ↑ 32%; (BOC)↑ 30% (TVR) | TVR and BOC: no dosage adjustment necessary; clinical and laboratory monitoring is recommended; discontinue tenofovir if adverse effects occur. |
| Protease inhibitor | |||||||
| Ritonavir (R) | ↓ 19% | ↓ 27% | ↓ 24% | ↓ 15% | – | – | TVR: no dosage adjustment necessary (with atazanavir, raltegravir or ritonavir). |
| Lopinavir+R | – | – | ↓ 32–54% | ↓ 53% | ↑ (Minimal) | – | TVR: combination should be avoided (with lopinavir, fosempravir or darunavir) due to HCV/HIV treatment failure.BOC: effects unknown. |
| Fosempravir+R | – | – | ↓ 32% | ↓ 33% | ↓ 47–49% (TVR) | ↓ 35–40% (TVR) | |
| Darunavir+R | – | – | ↓ 35% | ↓ 36% | ↓ 40–51% (TVR) | ↓ 40–47% (TVR) | |
| Atazanavir+R | – | – | ↓ 20% | ↓ 21% | ↑ 17% (TVR) | – | |
| Raltegravir | – | – | – | – | ↑ 31% (TVR) | – | |
| Antimicrobials | |||||||
| Rifampin | – | – | ↓ 92% | ↓ 86% | – | – | TVR and BOC: combination should be avoided. |
| Rifabutin | TVR and BOC: combination should be avoided. | ||||||
| Clarithromycin, erythromycin telithromycin | ↑ | ↑ | ↑ | TVR: caution is warranted and clinical monitoring is recommended.BOC: no dosage adjustment necessary. | |||
| Antifungals | |||||||
| Ketoconazole | ↑ 131% | ↑ 41% | ↑ 62% | ↑ 24% | ↑ 46–125% (TVR) | ↑ 23–75% (TVR) | BOC and TVR: doses of ketoconazole should not exceed 200mg/day. |
| Itraconazole | – | – | – | – | – | – | BOC and TVR: doses of itraconazole should not exceed 200mg/day. |
| Voriconazole | – | – | – | – | – | – | The interaction cannot be predicted. |
| Contraceptives | |||||||
| Drospirenone | – | – | – | – | ↑ 99% (BOC) | ↑ 57% (BOC) | BOC: combination should be avoided. |
| Ethinyl estradiol (EE) | – | – | – | – | ↓ 25% (BOTH) | – | TVR and BOC: non-hormonal contraception should be used. |
| Norethindrone | – | – | – | – | ↓ 11% (TVR) | – | TVR: reduces norethindrone slightly. |
| Anxiolytics/sleep aids | |||||||
| Midazolam oral | – | – | – | – | ↑ 796% (TVR)↑ 430% (BOC) | ↑177 (BOC)186% (TVR) | TVR and BOC: contraindicated. |
| Midazolam intravenous | ↑ 240% (TVR) | ↑ 2% (TVR) | TVR: contraindicated; BOC: halving the dose of intravenous midazolam could be considered with monitoring for toxic effects. | ||||
| Alprazolam | – | – | – | – | ↑ 35% (TVR) | – | TVR: a lower dose of intravenous alprazolam should be considered; closely monitor patient for respiratory depression and prolonged sedation. |
| Triazolam | TVR: contraindicated (oral and intravenous); BOC: contraindicated (oral). | ||||||
| Zolpidem | – | – | – | – | ↓ 42% (TVR) | – | TVR: a higher dose of zolpidem may be required; any dosage adjustments made during concomitant TVR therapy should be re-adjusted following completion of TVR therapy. |
| Antidepressants | |||||||
| Escitalopram | ↓ 35% (TVR);↓ 21% (BOC) | ↓ 30% (TVR) | |||||
| Immunosuppressants | |||||||
| Cyclosporine | – | – | – | – | ↑ 364% (TRV) | ↑ 32% (TRV) | BOC and TVR: empirically reducing the cyclosporine dose by 75%, then using therapeutic drug monitoring to further refine the cyclosporine dose and frequency; TVR ↑ the mean half life (12h from baseline to 53h). BOC: ↑ cyclosporine AUC (2.7-fold from baseline). |
| Tacrolimus | – | – | – | – | ↑ 6900% (TRV) | ↑ 835% (TRV) | TVR and BOC: significant dose reductions and prolongation of dosing interval may be needed; close monitoring recommended; TVR ↑ the mean half life (40h from baseline to 196h). BOC: ↑ tacrolimus AUC (17.1-fold from baseline). |
| Sirolimus | – | – | – | – | ↑ | ↑ | Sirolimus is expected to behave similarly to tacrolimus. |
| Dexamethasone, Prednisolone, methyl-prednisolone | ↓ | ↓ | – | – | TVR and BOC: co-administration with CYP3A4/5 inducers may decrease antiretrovirals levels; if possible, the combination should be avoided; used with caution if necessary. | ||
| Budesonide, Fluticasone (inhaled) | – | – | – | – | ↑ | – | BOC and TVR: if possible, the combination should be avoided; used with caution if necessary; may result in ↑ plasma concentrations of the steroid, causing significantly reduced serum cortisol. |
| Anticonvulsants | |||||||
| Carbamazepine | ↓ | – | ↓ | – | ↑ | – | TVR and BOC: if possible, the combination should be avoided; used with caution if necessary and dose titration is recommended. Concentrations of the anticonvulsant may be altered and concentrations of TVR and BOC may be decreased. |
| Phenobarbital | ↓ | – | ↓ | – | ↑ | – | |
| Phenytoin | ↓ | – | ↓ | – | ↑ | – | |
| Anti-psychotics | – | There are reports of anti-psychotic toxicity when combined with CYP3A inhibitor. | |||||
| Aripiprazole | – | – | – | – | ↑ | – | TRV and BOC: dosage of aripiprazole should be empirically reduced by half when are initiated and the anti-psychotic dose then titrated to effect. |
| Quetiapine | – | – | – | – | – | – | TRV and BOC: If possible, quetiapine use should be avoided. |
| Clozapine | – | – | – | – | – | – | TRV and BOC: monitor carefully for QT interval prolongation and discontinue clozapine if the QT interval exceeds 500ms. Patients who experience syncope, dizziness or palpitations should have further evaluation, including cardiac monitoring. |
| Pimozide | – | – | – | – | – | – | TVR and BOC: contraindicated. |
| HMG-CoA reductase inhibitors | |||||||
| Atorvastatin | – | – | – | – | ↑ 688% (TVR) | ↑ 960% (TVR) | TVR: contraindicated; BOC: ↑ Cmax by 2.7-fold and AUC by 2.3-fold; monitor patients for adverse effects (myopathy/rhabdomyolysis, elevated liver enzymes) and the lowest dose should be used (20mg atorvastatin). |
| Simvastatin | – | – | – | – | ↑ | ↑ | TVR: contraindicated. |
| Lovastatin | – | – | – | – | ↑ | ↑ | TVR: contraindicated. |
| Rosuvastatin | – | – | – | – | TVR and BOC: could be considered for use in combination with rosuvastatin; has not been studied to date. | ||
| Angiotensin II receptor blocker | |||||||
| Irbesartan, Losartan | – | – | – | – | ↑ | – | TVR and BOC: dose reductions could be considered. |
| β-Blockers | |||||||
| Carvedilol, Nabivolol | – | – | – | – | ↑ | – | TVR and BOC: dose reductions could be considered. |
| Calcium channel blockers | |||||||
| Felodipine, nifedipine, nicardipine, nisoldipine | – | – | – | – | – | – | TRV and BOC: clinical monitoring for adverse effects is recommended (headache, peripheral edema, hypotension, tachycardia). |
| Amlodipine | – | – | – | – | ↑ 179% (TVR) | ↑ 27% (TVR) | TVR: dose reductions could be considered in patients initiating antivirals; if dose adjustments are required, return to normal amlodipine dosing after therapy with telaprevir has been completed. |
| Verapamil, diltiazem | – | – | – | – | ↑ | ↑ | TRV: caution and clinical monitoring advised. |
| Antiarrhythmics | |||||||
| Amiodarone, bepridil, quinidine, flecainide, propafenone | – | – | – | – | ↑ | ↑ | TVR and BOC: caution and clinical monitoring is recommended; potential for serious and/or life-threatening adverse events. |
| Other agents | |||||||
| Digoxin | – | – | – | – | ↑ 85% (TVR) | ↑ 50% (TVR) | TVR and BOC: initial dose should be used with titration and monitoring of serum digoxin concentrations. |
| Alfuzosin | – | – | – | – | – | – | TVR: contraindicated; co-administration may result in which may result in hypotension. |
| PDE-5 inhibitors (sildenafil, tadalafil, vardenafil) | – | – | – | – | ↑ | – | TVR and BOC: contraindicated (for pulmonary arterial hypertension); lower doses should be used; for erectile dysfunction increased monitoring for adverse events (hypotension, visual abnormalities, syncope, and priapism). |
| Colchicine | – | – | – | – | ↑ | – | TVR and BOC: a reduction in colchicine dosage or an interruption of colchicine treatment (TVR) is recommended; avoid co-administration in renal/hepatic impairment. Significant increases in colchicine levels expected with strong CYP3A4 inhibitors; fatal colchicine toxicity reported. |
| Warfarin | – | – | – | – | ↑ or ↓ | – | TVR and BOC: INR must be monitored closely. |
| Ergot alkaloids | TVR and BOC: contraindicated due to the potential for acute ergot toxicity (e.g., peripheral vasospasm, ischemia of the extremities and/or other tissues) | ||||||
| Hypericum perforatum | ↓ | – | ↓ | – | – | – | TVR and BOC: contraindicated. |
(↑) increase; (↓) decrease; (–) no effect or unknown.
BOC is metabolized by aldoketoreductase (AKR) 1C2 and 1C3 and, to a lesser extent, by oxidative metabolism mediated by CYP3A4/5. As BOC utilizes multiple routes of metabolism, it is less prone to drug interactions.31,32 The primary route of metabolism of TVR is CYP3A4. This drug has low potential to induce CYP2C, 3A, or 1A.
In addition to interactions mediated by CYP3A, TVR and BOC are susceptible to interactions membrane transporter-mediated. Both agents are substrates and inhibitors of P-glycoprotein (P-gp) and can inhibit or saturate this transporter, thus increasing the concentrations of substrates.29,31,32Table 4 lists the main substrates, inhibitors and inducers of the CYP3A4.
Main substrates, inducers and inhibitors of cytochrome P450 3A4.
| Substrates | Inducers | Inhibitors | ||||||
|---|---|---|---|---|---|---|---|---|
| Alfentanil | Clarithromycin | Ergotamine | Indinavir | Omeprazole | Simvastatin | Aminoglutethimide | Amiodarone | Nifedipine |
| Alfuzosin | Clindamycin | Erlotinib | Isradipine | Ondansetron | Sirolimus | Amprenavir | Amprenavira | Nilotinib |
| Aliskiren | Clomipramine | Erythromycin | Itraconazole | Oral Contraceptives | Solifenacin | Aprepitant | Aprepitanta | Norfloxacin |
| Almotriptan | Clonazepam | Escitalopram | Ketoconazole | Oxybutynin | Sorafenib | Carbamazepine | Atazanavirb | Pomegranate |
| Alprazolam | Clopidogrel | Esomeprazole | Lapatinib | Paclitaxel | Sunitinib | Dexamethasone | Chloramphenicol | Posaconazoleb |
| Amitriptyline | Clozapine | Estrogens, oral | Lansoprazole | Pantoprazole | Tacrolimus | Efavirenz | Cimetidine | Pazopanib |
| Amiodarone | Cocaine | Contraceptives | Letrozole | Pazopanib | Tadalafil | Ethosuximide | Ciprofloxacin | Prednisone |
| Amlodipine | Colchicine | Eszopiclone | Levobupivacaine | Pimozide | Tamoxifen | Etravirine | Clarithromycinb | Propoxyphene |
| Amprenavir | Cyclobenzaprine | Ethinyl estradiol | Lidocaine | Pioglitazone | Telithromycin | Garlic supplements | Cyclosporine | Quinine |
| Aprepitant | Cyclophosphamide | Ethosuximide | Lopinavir | Prednisolone | Temazepam | Glucocorticoids | Danazol | Ranolazine |
| Asenapine | Cyclosporine | Etonogestrel | Loratadine | Prednisone | Testosterone | Glutethimide | Delavirdine | Ritonavirb |
| Atazanavir | Dapsone | Etoposide | Losartan | Progesterone/ | Tiagabine | Griseofulvin | Diltiazema | Saquinavirb |
| Atorvastatin | Darifenacin | Etravirine | Lovastatin | Progestins | Tinidazole | Modafinil | Darunavir/ritonavirb | Synercid |
| Beclomethasone | Darunavir | Exemestane | Maraviroc | Propafenone | Tipranavir | Nafcillin | Dronedaronea | Telithromycinb |
| Bepridil | Dasatinib | Everolimus | Methadone | Propoxyphene | Tolterodine | Nevirapine | Erythromycina | Tipranavir/ritonavirb |
| Bexarotene | Delavirdine | Felodipine | Methylprednisolone | Quetiapine | Tolvaptan | Oxcarbazepine | Ethinyl estradiol | Verapamila |
| Bromocriptine | Desogestrel | Fentanyl | Miconazole | Quinidine | Toremifene | Phenobarbital | Everolimus | Voriconazoleb |
| Budesonide | Dexamethasone | Fesoterodine | Midazolam | Quinine | Tramadol | Phenytoin | Fluconazolea | Zafirlukast |
| Buprenorphine | Dextromethorphan | Fexofenadine | Mifepristone | Rabeprazole | Trazodone | Primidone | Fluoxetine | |
| Buspirone | Diazepam | Finasteride | Mirtazapine | Ramelteon | Triazolam | Rifabutin | Fluvoxamine | |
| Busulfan | Dihydroergotamine | Flutamide | Modafinil | Ranolazine | Trimetrexate | Rifampin | Fosamprenavira | |
| Cannabinoids | Diltiazem | Fluticasone | Mometasone | Repaglinide | Valdecoxib | Rifapentine | Grapefruita | |
| Caffeine | Disopyramide | Fluvestrant | Montelukast | Romidepsin | Vardenafil | Ritonavir | Indinavirb | |
| Carbamazepine | Docetaxel | Galantamine | Nateglinide | Rifabutin | Verapamil | St. John's wort | Imatinib | |
| Cevimeline | Dofetilide | Guanfacine | Nefazodone | Rifampin | Vinblastine | Isoniazid | ||
| Chlorpheniramine | Dolasetron | Haloperidol | Nelfinavir | Ritonavir | Vincristine | Itraconazoleb | ||
| Cilostazol | Domperidone | Hydrocodone | Nevirapine | Salmeterol | Vinorelbine | Ketoconazoleb | ||
| Ciclesonide (desciclesonide | Donepezil | Hydrocortisone | Nicardipine | Saquinavir | Voriconazole | Lapatinib | ||
| [active Metabolite]) | Doxorubicin | Ifosfamide | Nifedipine | Saxagliptin | (r)-warfarin | Methylprednisolone | ||
| Cinacalcet | Dronabinol | Iloperidone | Nilotinib | Sertraline | Zaleplon | Mifepristone | ||
| Cisapride | Dronedarone | Imatinib | Nimodipine | Sibutramine | Zileuton | Nefazodoneb | ||
| Citalopram | Dutasteride | Imipramine | Nisoldipine | Sildenafil | Ziprasidone | Nelfinavirb | ||
| Efavirenz | Ixabepilone | Nitrendipine | Silodosin | Zolpidem | Nicardipine | |||
| Eplerenone | Norethindrone | Zonisamide | ||||||
Italics denote those substrates, inhibitors, and inducers that have been involved in a drug interaction of clinical relevance, and/or are associated with strong drug interaction warnings or recommendations for specific intervention (i.e., dose alteration, laboratory monitoring, or avoidance). For many medications, strength of inhibition in vivo is undetermined.
Several commonly used antiretroviral drugs to treat HIV affect the CYP3A metabolic pathway or are its substrates.31,33 Concomitant administration of TVR and efavirenz resulted in reduced steady-state exposure of both drugs. However, by increasing the TVR dose from 800mg to 1125mg every 8h (i.e. 50%), TVR exposure can be at least partially compensated. This strategy has been successfully used in a phase 2a study in HIV/HCV coinfected patients.29,32
Ritonavir is used at a low dose (100mg once or twice daily) to inhibit CYP3A metabolism of other HIV PIs and enhance its levels. This strategy was investigated for both BOC and TVR. However, ritonavir-boosting does not appear to decrease TVR or BOC pill burden or dosing frequency.29,31
In clinical studies, administration of TVR with ritonavir-boosted HIV PIs atazanavir, darunavir, fosamprenavir and lopinavir decreased the exposure to TVR. This effect was not predicted, as HIV PIs boosted with ritonavir inhibit CYP3A4 and therefore may be expected to increase TVR levels.29,32
Ritonavir-boosted atazanavir is the combination less affected and is being studied in HIV/HCV coinfected patients without dose adjustment of either agent.29,31
Raltegravir is an attractive agent for use in the treatment of HIV in the HIV/HCV coinfected patients as its primary route of metabolism is glucuronidation and it does not inhibit or induce CYP enzymes. In combination with TVR, raltegravir AUC was increased, presumably due to TVR inhibition of P-gp. Raltegravir has a wide therapeutic index and this increase in AUC is not expected to have clinical relevance.29 The risk of anemia is higher if zidovudine is used with BOC or TVR, particularly in combination with RBV.32,33
ImmunosuppressantsIt is critical to determine the safest and most effective doses of TVR or BOC to HCV-transplanted patients. However, most commonly used immunosupressors affect the CYP 3A metabolic pathway or are themselves substrates. These agents have narrow therapeutic windows and the impact of introducing an agent with potential interaction such as BOC or TVR, may result in serious AEs.31 Preliminary data suggest that cyclosporine may be preferred to tacrolimus in the setting of TVR or BOC-based HCV treatment, but it may still be possible to use tacrolimus in a very controlled manner.29
Sirolimus is expected to behave similarly to tacrolimus, but drug interaction with DAAs has not been studied yet.29 The longer half-life of sirolimus (60h), together with the significant anemia caused by BOC or TVR could provide additive toxicity and make concurrent therapy difficult to manage in clinical practice.31
HMG-CoA reductase inhibitorsSimvastatin, atorvastatin and lovastatin are highly dependent on CYP3A for its metabolism and increasing reports alert to the risk of myopathy and rhabdomyolysis in patients with higher concentrations of simvastatin and atorvastatin caused by drug interactions with a potent CYP3A inhibitor.29,34
Pravastatin is metabolized by multiple pathways. The mechanism for the interaction of BOC with pravastatin is unclear, but may be related to BOC related inhibition of OATP1B1.29
Rosuvastatin is metabolized by CYP2C9 and 2C19 and could be considered for use in combination with BOC or TVR, but it still needs to be confirmed as unexpected increases in rosuvastatin concentrations have been reported in combination with several HIV PIs.29
Oral contraceptivesRBV is highly teratogenic and prevention of pregnancy during its use is critical. Patients are advised to use at least two forms of birth control during treatment with Peg-IFN/RBV and for six months thereafter.29,35
The combination of BOC and TVR and ethinylestradiol may decrease the plasma concentrations of the drug with potential for failure of birth control. Systemic hormonal contraception must be augmented by two alternative effective forms of contraception and may include intrauterine devices and barrier methods during therapy and for six months following BOC therapy.36 BOC increases drospirenone levels and high concentrations of this drug can theoretically cause hyperkalemia.29 In addition, based on a recent review by the FDA, drospirenone-containing birth control pills may be associated with higher risk for blood clots than other progestin-containing pills.34
Psychotropic medicationsThe selective serotonin reuptake inhibitors (SSRIs) are generally the first line treatment of depression in HCV patients due to their safety in overdose and improved tolerability. There are no obvious concentration-effect data for the SSRIs, so it is unknown if reductions in exposures can be translated in reduced ability to control depressive symptoms.29
There are no formal drug interaction studies between TVR or BOC and antipsychotics, thus predictions must be made based on knowledge of the clinical pharmacology of each agent.29
Midazolam is a selective CYP3A substrate. Flurazepam, quazepam and triazolam are also highly dependent on CYP3A for metabolism and combination with TVR or BOC should be avoided.29 Lorazepam and oxazepam are not converted into active metabolite and are only conjugated, thus these drugs could be considered for patients using IPs. Trazodone is also used as a sleep aid. With the HIV PI ritonavir, trazodone exposures are increased, causing nausea, dizziness, hypotension and syncope.29
Cardiovascular drugsCYP enzymes are not involved in the metabolism of ACE inhibitors or diuretics. Among the beta blockers, only carvedilol and nabivolol are metabolized to some extent by CYP3A4. There is a contribution of CYP3A4 to the metabolism of angiotensin II receptor blockers irbesartan and losartan. The calcium channel blockers are highly dependent on CYP3A for metabolism and are therefore susceptible to increases in exposure with BOC or TVR.29
BOC and TVR are both substrates and inhibitors of P-gp. Digoxin is not metabolized, but is a selective substrate of P-gp. TVR can increase the exposure of digoxin and it was thought to result from inhibition or saturation of P-gp in the gut rather than at a systemic level.29,32
Amiodarone is a substrate of many CYP enzymes, but the main isoenzyme is CYP 3A4. It is advised to monitor amiodarone plasma concentrations and the patient for adverse effects related to amiodarone, including nausea, vomiting, visual changes, and cardiac arrhythmias if coadministration with BOC is required.36
Antimicrobials and antifungalsKetoconazole, itraconazole and voriconazole are strong CYP3A inhibitors (>5-fold increase in exposure, or >80% decrease in clearance of substrate). Fluconazole is a moderate inhibitor (2- to <5-fold increase in exposure or 50–80% decrease in clearance of substrate).37 Conversely, ketoconazole and itraconazole are metabolized only by CYP 3A4, fluconazole is not metabolized by CYP and voriconazole is substrate of CYP 2C9, 2C19 and 3A4.
Other agentsAlthough the interaction between colchicine and BOC or TVR has not been studied yet, an interaction between colchicine and clarithromycin, a strong CYP3A4 inhibitor, resulted in fatal colchicine toxicity. It is advised to treat the gout flare with reduced colchicine dose to 0.6mg tablet for one dose, followed by 0.3mg one hour later, with the repeated dose no earlier than 3 days. For prophylaxis of gout flare, reduction of colchicine from an original dose of 0.6mg twice daily to 0.3mg once daily or from an original dose of 0.6mg once daily to 0.3mg once every other day is recommended. For the treatment of familial Mediterranean fever, the maximum daily colchicine dose should be no more than 0.6mg daily (0.3mg twice daily).36
Coadministration of BOC or TVR and domperidone may result in significantly increased plasma concentrations of domperidone, with risk of serious cardiac events (ventricular arrhythmias and sudden cardiac death). Case–control studies have demonstrated an association of serious ventricular arrhythmias and sudden cardiac death, particularly with domperidone doses greater than 30mg/day and in patients older than 60 years. Domperidone should be initiated at the lowest possible dose and titrated with caution. It must be discontinued in the presence of dizziness, palpitations, syncope, or seizure. Dosage adjustments made during concomitant use of TVR need readjusts after TVR therapy is completed.36
Conflict of interestThe authors have no conflict of interest to declare.