Crimean-Congo hemorrhagic fever (CCHF) is a severe, often potentially fatal disease in humans, caused by infection with CCHF virus (CCHFV) belonging to the genus Nairovirus of the Bunyaviridae family. The current approach to treatment of CCHF is based on supporting hematologic and coagulation status of the patient, with replacement of cells and factors as needed, but use of ribavirin is controversial. In this study it was aimed to evaluate and compare outcomes of pediatric patients receiving or not receiving ribavirin.
The study included patients younger than 18 years of age admitted to Ankara Hematology Oncology Children's Training and Research Hospital between January, 2009, and November,
2014, with fever, bleeding, leukopenia, and/or thrombocytopenia with positive IgM and/or PCR results for CCHFV in blood. Physical findings and the laboratory tests were included in data analysis.
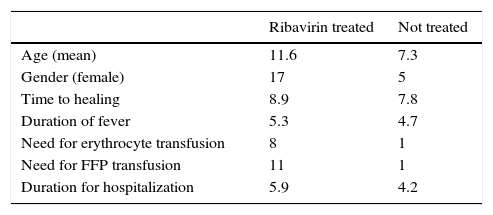
Out of 46 pediatric patients (18 female, 28 male), aged between 1 and 17 years (mean: 10.1±4.8 years) with CCHF, 30 patients were treated with ribavirin, characteristics of the patients are presented in Table 1.
Diagnosis of CCHF was confirmed by PCR in 32 (69.6%) patients, by serology in 16 (42.1%) patients, both serology and PCR in 2 patients. On admission anemia was present in 11 (23.9%) patients, leukocytopenia in 39 (84.8%) patients, thrombocytopenia in 40 (86.9%) patients. Elevated AST, ALT, LDH, and CPK levels were present in 38 (82.6%), 35 (76.1%), 40 (87%), 25 (54.3%) patients, respectively.
The length of stay in hospital ranged from 1 to 14 days (mean: 5.4±3.02 days). No fatal cases were observed. In patients treated with ribavirin, duration of therapy ranged from 3 to 10 days (mean: 8.7±2.01 days).
The only currently used antiviral drug in CCHF is ribavirin; however, its efficacy is a matter of concern as randomized controlled trials are lacking. In our study both groups treated and not treated with ribavirin presented no fatal cases. Also in ribavirin treated group need for erythrocyte and plasma transfusion was more frequent and duration of fever and hospitalization were longer in ribavirin treated group but this could have been due to ribavirin use in more severe patients.
In our previous study we showed that Turkish pediatric patients had a milder course of CCHF,1 which might be due to a lower cytokine response in pediatric cases. Lower cytokine levels in pediatric patients in could be a good prognostic factor for a favorable outcome.2
One randomized controlled trial evaluating the efficacy of ribavirin performed in
Turkey, compared 64 patients who received ribavirin and 72 who did not showed no significant difference in mortality, proportion of patients requiring platelet transfusion, length of hospital stay, recovery time, or laboratory parameters.3 However, that study included late cases and ribavirin might not be beneficial in late stages of the disease. Side effects of ribavirin are the major obstacle, such as bradycardia, genotoxicity, and mutagenicity.4–7
As the use of ribavirin in CCHF is still controversial8,9 more data are warranted to reach a definitive conclusion.
Conflicts of interestThe authors declare no conflicts of interest.