Pseudomonas aeruginosa is an opportunistic pathogen of both community-acquired and hospital-acquired infections which rarely causes disease in healthy immunocompetent individuals. The aim of the study was to explore total plasmid contents, antibiotic susceptibility profiles and heterogeneity features of the isolates with a combined approach of Enterobacterial Repetitive Intergenic Consensus-Polymerase Chain Reaction (ERIC-PCR) and Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) profiles.
A total of 54 P. aeruginosa isolates were collected from various clinical specimens (urine 32%, sputum 17%, wound 15%, blood 13%, respiratory secretion 9%, aspirate 6%, abscess 2%, endotracheal aspirate 2%, pleural fluid 2%, seminal fluid 2%) of hospitalized people in Sinop and nearby cities, Turkey. Age of the hospitalized individuals ranged from 0.6 to 91 years (mean±SD: 59.95±23.09 years). Reference strain P. aeruginosa ATCC 27853 was used as a positive control for all physiological and biochemical tests, as well as molecular tests. All tests were performed according to Bergey's manual of systematic bacteriology.1
Antimicrobial susceptibility testing of 19 antibiotics was carried out according to Clinical and Laboratory Standards Institute (CLSI) recommendation. All the strains tested were resistant to sulfametoxazole/trimethoprim, tetracycline, chloramphenicol, penicillin G, and clindamycin and highly resistant to ampicillin/sulbactam, colistine, and cefotaxime. Gentamicin, imipenem, ciprofloxacin, and ceftazidime were chosen to determine multidrug resistance (MDR) and 31.4% (n=17) of the isolates were defined as MDR. MDR P. aeruginosa rates are reported in the range of 0.6–32% according to the geographic location and surveillance study types.2 Based on this data, the values of MDR P. aeruginosa in our study seem close to the highest value.
Plasmid DNA and chromosomal DNA isolation was performed according to the method of Sambrook et al.3 with some modifications. Plasmid profiling demonstrated that all clinical isolates harbored between 3 and 6 plasmids with molecular weights ranging between 22,000bp and 600bp and plasmids of 14,500bp, 1392bp, and 983bp were detected in all strains isolated.
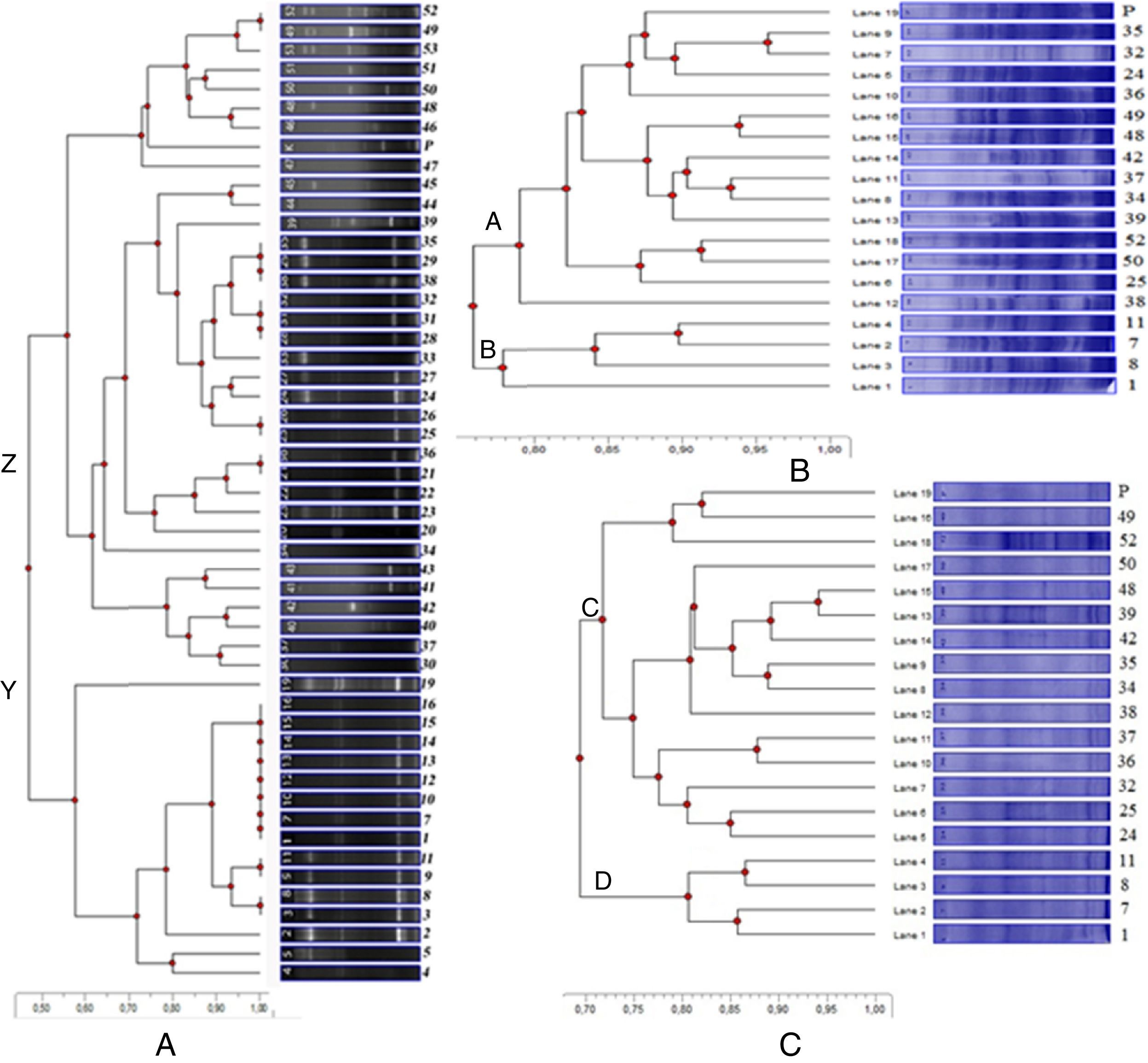
ERIC-PCR and investigation of the prevalence of virulence genes algD, exoS, lasB, toxA, and rhlAB were performed by previously described primers and conditions4 with some modifications. There were 3–12 bands with molecular weights ranging from 120bp to 14,750bp generated by ERIC primers for a total of 50 strains and reference strain. In the dendrogram, the isolates were separated into two major clusters (Z and Y) at similarity levels between 47% and 100% (Fig. 1A). The virulence genes toxA, lasB, exoS, algD, and rhlAB were present in 36 (66.6%), 53 (98.1%), 27 (50%), 47 (87%), and 52 (96.2%) of the isolates, respectively. So, the clinical isolates proved to harbor the virulence genes investigated at 50% or above.
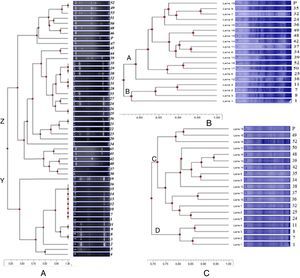
Extractions of whole cell and extracellular proteins for a total of 18 clinical isolates and reference strain were carried out with the methods of Laemmli5 and Wessel and Flügge.6 respectively. Protein analysis was carried out with SDS-PAGE as described by Laemmli.5 Whole cell and extracellular protein bands ranged from 200kDa to 30kDa and 197kDa to 54kDa, respectively. In the dendrograms the isolates were separated into two major clusters (A and B) between 76% and 96% (Fig. 1B) and (C and D) between 69% and 94% (Fig. 1C) for whole cell proteins and extracellular proteins, respectively.
The less discriminative power of SDS-PAGE at strain level may inevitably demand to benefit from the advantages provided by specialized molecular tools alone or in combination. ERIC-PCR is an appropriate, inexpensive, fast, reproducible and discriminatory DNA typing tool for effective epidemiological surveillance of P. aeruginosa isolates. Conventional antibiotics fail to effectively clear chronic infections caused by biofilms and necessitate new measures as an approaching step to post-antibiotic era. Inhibition of quorum-sensing and related virulence factors of P. aeruginosa thus may be a promising approach to combat with the pathogenicity and elimination of the infections.
Conflicts of interestThe authors declare no conflicts of interest.