Corynebacterium spp. are becoming recognized as pathogens that potentially cause various infections. We aimed to evaluate the clinical characteristics associated with Corynebacterium spp. bacteremia.
Patients and methodsWe retrospectively reviewed the medical records of all adult patients who had positive blood cultures for Corynebacterium spp. in a single university hospital between January 2014 and December 2016. Patients were divided into a bacteremia group and a contamination group based on microbiological test results and clinical characteristics. Patients’ characteristics, antimicrobial susceptibility of isolated species, antimicrobials administered, and patient outcomes were evaluated.
ResultsCorynebacterium spp. were isolated from blood samples of 63 patients; Corynebacterium striatum was the predominant isolate. Twenty-eight patients were determined to have bacteremia. Younger age (p=0.023), shorter time to positivity (p=0.006), longer hospital stay (p=0.009), and presence of an indwelling vascular catheter (p=0.002) were observed more often in the bacteremia group compared to the contamination group. The source of infection in most patients with bacteremia was an intravenous catheter. All tested strains were susceptible to vancomycin. Four of the 27 patients with bacteremia died, despite administration of appropriate antimicrobial therapy.
ConclusionsWe found that younger age, shorter time to positivity, and presence of an indwelling catheter were related to bacteremia caused by Corynebacterium spp. Appropriate antimicrobials should be administered once Corynebacterium spp. are isolated from the blood and bacteremia is suspected.
Except for Corynebacterium diphtheriae, all Corynebacterium spp. are ubiquitous skin commensals and are usually considered to be blood culture contaminants.1 However, some reports have highlighted the importance of C. striatum and C. jeikeium as causes of catheter-related bloodstream infections in immunocompromised patients, and as causes of various infections in immunocompetent patients, such as arthritis and endocarditis.2–5 It is difficult to distinguish contamination from bacteremia when Corynebacterium spp. or coagulase-negative staphylococci are isolated from blood samples.6–9 In clinical practice, it is important to distinguish contamination from bacteremia to prevent unnecessary prescription of antimicrobial agents, which can lead to selection of antimicrobial-resistant organisms, longer duration of hospitalization, and increased costs.10
There are few studies on Corynebacterium spp. infection, and the clinical significance of these organisms remains unclear. Thus, in this study, we aimed to evaluate the clinical characteristics of bloodstream infections caused by Corynebacterium spp. by analyzing clinical cases of blood cultures positive for Corynebacterium spp.
Patients and methodsPatient selection and study periodThis retrospective cohort study was conducted at the Nihon University Itabashi Hospital (NUIH), a 1037-bed teaching hospital. The catchment area of NUIH encompasses the north part of Tokyo, Japan. We reviewed the medical records of all patients aged >18 years who had positive blood culture results for Corynebacterium spp. between January 2014 and December 2016. Only the first episode was considered in case of multiple episodes within a 4-week period.
DefinitionsBlood specimens were cultured using a set of culture bottles (BACTEC 92F and 93F; BD Diagnostic Systems, Franklin Lakes, NJ, USA) and automated systems (BACTEC 9240 and 9120; BD Diagnostic Systems) with continuous agitation were used. Bacterial species were identified through several methods, including assessment of morphologic appearance and colonies, and assessment of biochemical characteristics using manual procedures or commercial kits (API series; bioMérieux, Marcy l’Etoile, France and N-ID test SP-18; Nissui Pharmaceutical Co., Ltd., Tokyo, Japan) or an automated identification system (RAISUS system, Nissui Pharmaceutical Co., Ltd.). Antimicrobial susceptibility tests were performed by broth microdilution using the Clinical and Laboratory Standards Institute's breakpoints.11
Patients were categorized as having bacteremia if at least two blood culture sets taken at the same time turned out positive for the same Corynebacterium spp., or when one blood culture specimen and another clinically relevant sample taken from another site (such as a catheter tip, sputum, or pus) yielded positive results. If only one set of blood cultures turned out positive and culture specimens taken from other sites were negative, or if another infection was more likely at the time, the case was deemed to be contamination. The final decision of bacteremia or contamination was determined by the authors, which includes certified infectious diseases specialists on the antimicrobial stewardship program team.
Evaluated parametersWe reviewed the medical records to extract data on each patient's background (age, sex, underlying disease, body temperature, clinical department, presence of a vascular catheter, laboratory data), clinical course (response to antimicrobial therapy), and outcome. In addition, we analyzed microbiological data, including time from admission to collection of blood culture samples and time to positivity; antimicrobial susceptibility test results; and isolation of bacteria from sites other than blood.
Statistical analysisContinuous data were expressed as the median and interquartile range (IQR), and categorical variables as percentages or absolute values. Statistical significance was calculated using the chi-square test for categorical variables and the Mann–Whitney U test for continuous variables. A p-value <0.05 was considered statistically significant. Statistical analyses were performed using StatMate V software (ATMS Co., Ltd., Tokyo, Japan).
Ethical considerationsThe Clinical Research Judging Committee at NUIH approved the study protocol and waived the need for informed consent given the retrospective nature of the study.
ResultsDuring the study period, 66 patients had positive blood cultures for Corynebacterium spp. Three patients were excluded because of a second episode of serial infection. Of the remaining 63 patients, 28 (44%) met the criteria for bacteremia and 35 (56%) met the criteria for contamination. Patients’ baseline characteristics are summarized in Table 1. The overall median age was 73 years, and the contamination group was significantly older than the bacteremia group (p=0.023). Men constituted 74.6% of cases. Fourteen cases were identified in the emergency department and critical care medicine; this department had a significantly higher proportion of contamination cases than did the other departments (p=0.049). Overall, 39 patients had a central venous catheter in place, five had a peripheral venous catheter in place, and 19 did not have a venous catheter; bacteremia was diagnosed more frequently in patients who had peripheral or central venous inserted catheters (p=0.002). The median interval from admission to blood culture sampling was 25 days (IQR, 6–49); this interval was significantly longer in the bacteremia group than in the contamination group (p=0.006). The median time to culture positivity was significantly shorter in the bacteremia group than in the contamination group (p=0.009). There were no significant differences in median leukocyte count or C-reactive protein level between the two groups. In 51 of the 63 cases, the patient had fever (temperature >38°C) at the time the blood culture sample was taken.
Characteristics of patients with bacteremia or blood culture contamination with Corynebacterium spp.
| Total | Bacteremia | Contamination | p-value | |
|---|---|---|---|---|
| Number of cases, n (%) | 63 | 28 | 35 | |
| Age (years), median (IQR) | 73 (62.0–81.5) | 65.5 (57.3–77.0) | 75 (70.0–83.0) | 0.023 |
| Male:female ratio | 47:16 | 22:6 | 25:10 | 0.517 |
| Department, n (%) | ||||
| Emergency and critical care medicine | 14 (22) | 3 (11) | 11 (31) | 0.049 |
| Gastrointestinal medicine | 8 (13) | 4 (14) | 4 (11) | |
| Hematology and rheumatology | 8(13) | 6 (21) | 2 (6) | |
| Cardiovascular surgery | 7 (11) | 5 (18) | 2 (6) | |
| Nephrology | 5 (8) | 4 (14) | 1 (3) | |
| Cardiovascular medicine | 5 (8) | 2 (7) | 3 (9) | |
| Digestive surgery | 4 (6) | 2 (7) | 2 (6) | |
| General medicine | 3 (5) | 0 (07) | 3 (9) | |
| Othera | 9 (14) | 2 (7) | 7 (20) | |
| Underlying diseases, n | ||||
| Malignancy | 22 | 13 | 9 | 0.086 |
| DM | 6 | 4 | 2 | 0.249 |
| ESRD on dialysis therapy | 12 | 8 | 4 | 0.085 |
| LC | 3 | 2 | 1 | 0.427 |
| Vascular catheter placement, n (%) | ||||
| CV catheter | 39 (62) | 21 (75) | 18 (51) | |
| Peripheral catheter | 5 (8) | 4 (14) | 1 (3) | |
| No catheter | 19 (30) | 3 (11) | 16 (46) | 0.002 |
| Time from admission to sampling (days), median (IQR) | 25 (6–49) | 36 (15–61) | 13 (0–31) | 0.006 |
| Time to positive culture detection (days), median (IQR) | 1 (1–2) | 1 (1–1) | 2 (1– 2) | 0.009 |
| Temperature >38°C, n (%) | 51 (81) | 25 (89) | 26 (74) | 0.132 |
| WBC count (×109/L) | 9.2 (5.0–13.2) | 8.9 (3.1–12.5) | 9.2 (7.3–13.8) | 0.290 |
| CRP (mg/dL), median (IQR) | 6.6 (3.3–15.9) | 6.1 (3.2–13.5) | 7.1 (4.4–16.4) | 0.481 |
| Hospital stay (days), median (IQR) | 57 (27–97) | 81 (38–135) | 36 (17–63) | 0.002 |
Abbreviations: IQR, interquartile range; DM, diabetes mellitus; ESRD, end-stage renal disease; LC, liver cirrhosis; CV, central venous; WBC, white blood cell; CRP, C-reactive protein.
Final identification of the Corynebacterium spp. isolated were C. striatum (n=38), C. jeikeium (n=6), and C. argentoratense (n=2). The species was reported only as “Corynebacterium sp.” in 19 cases, either because the physician in charge did not request further identification or the laboratory was unable to identify the species. Two species (C. striatum and Corynebacterium sp.) were identified simultaneously in two cases (Table 2). There was a significant difference between the different species regarding rates of bacteremia versus contamination (p=0.001). Two sets of blood cultures were obtained from 60 patients, and one set of blood cultures was obtained from three patients. Multiple blood culture specimens were positive in 38% (23/60) of these patients. The same organism isolated from blood samples was isolated from venous catheter tip specimens (n=11), followed by sputum, wound site, urine, and drain specimens. In 45 patients, only the blood culture specimen was positive for Corynebacterium spp. A central or peripheral venous catheter was considered the source of infection in 20 of the 28 patients with bacteremia; the catheter tip was culture positive for Corynebacterium spp. in 12 cases and culture negative in three cases; the catheter was not removed and sent for culture in the remaining five cases.
Final identification of Corynebacterium spp., blood culture positivity pattern, and sites from which specimens, taken simultaneously with blood specimens, cultured positive for Corynebacterium spp.
| Total (n=63) | Bacteremia (n=28) | Contamination (n=35) | |
|---|---|---|---|
| Identified speciesa | |||
| Corynebacterium striatum | 38 | 22 | 16 |
| Corynebacterium jeikeium | 6 | 4 | 2 |
| Corynebacterium argentoratense | 2 | 1 | 1 |
| Corynebacterium sp. | 19 | 1 | 18 |
| Positivity [positive set(s)/set(s) taken] | |||
| 2/2 | 23 | 23 | 0 |
| 1/2 | 39 | 5 | 34 |
| 1/1 | 3 | 0 | 3 |
| Isolation sitesb | |||
| Catheter tip | 11 | 9 | 2 |
| Sputum | 5 | 2 | 3 |
| Wound (pus) | 3 | 2 | 1 |
| Urine | 2 | 2 | 0 |
| Drain | 1 | 1 | 0 |
| None | 45 | 15 | 30 |
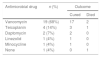
The results of the antimicrobial susceptibility tests are shown in Table 3. All isolates were susceptible to minocycline, vancomycin, and teicoplanin. Most isolates were resistant to penicillin, imipenem/cilastatin, erythromycin, clindamycin, and levofloxacin. Table 4 shows the antimicrobial agents administered after the final report was issued by the microbiology laboratory. Appropriate antimicrobials were administered in all except one case in which the patient died before the final report was issued. Of the 27 patients who received appropriate antimicrobial therapy, 24 were cured while three died as a result of Corynebacterium bacteremia.
Antibiotic susceptibility of Corynebacterium spp. isolated from blood culture specimens (susceptible/tested (%)).
| ABPC | ABPC/SBT | IPM/CS | GM | EM | CLDM | MINO | VCM | TEIC | ST | LVFX | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| C. striatum (n=31) | 2/31 (7) | 5/31 (16) | 5/31 (16) | 22/22 (100) | 3/22 (14) | 3/31 (10) | 31/31 (100) | 31/31 (100) | 31/31 (100) | 27/31 (87) | 1/31 (3) |
| C. jeikeium (n=6) | 0/6 (0) | 0/6 (0) | 1/6 (17) | 1/6 (17) | 0/6 (0) | 0/6 (0) | 4/6 (67) | 6/6 (100) | 6/6 (100) | 4/6 (67) | 0/6 (0) |
| C. argentoratense (n=2) | 0/2 (0) | 0/2 (0) | 0/2 (0) | 2/2 (100) | 2/2 (100) | 2/2 (100) | 2/2 (100) | 2/2 (100) | 2/2 (100) | 2/2 (100) | 0/2 (0) |
| Corynebacterium sp. (n=1) | 0/1 (0) | 0/1 (0) | 1/1 (100) | 1/1 (100) | 0/1 (0) | 0/1 (0) | 1/1 (100) | 1/1 (100) | 1/1 (100) | 0/1 (0) | 0/1 (0) |
Abbreviations: ABPC, ampicillin; ABPC/SBT, ampicillin–sulbactam; IPM/CS, imipenem/cilastatin; GM, gentamicin; EM, erythromycin; CLDM, clindamycin; MINO, minocycline; VCM, vancomycin; TEIC, teicoplanin; ST, sulfamethoxazole–trimethoprim; LVFX, levofloxacin.
In this retrospective study, we analyzed the clinical data of patients who had Corynebacterium spp. identified on blood culture. More than 40% of patients were determined to have bacteremia based on their clinical characteristics and course. C. striatum was the species most frequently identified in patients with bacteremia. The route of infection was via venous catheters in >50% of patients with bacteremia; this infection route has been reported in several previous studies.2,7,12 Regarding intravascular catheter-related infections, there is broad consensus that growth of the same organism in paired blood culture samples, drawn from a peripheral vein and the suspected source, indicates a true or relevant infection.13Corynebacterium spp. can colonize prostheses, catheter tips, and ventilator and feeding tubes; however, Corynebacterium spp. isolated from these prostheses are usually considered contaminants. In general, bacterial skin commensals have relatively low virulence, but De Souza et al.14 recently reported that biofilm production by antimicrobial-resistant C. striatum is a new virulence factor and is related to nosocomial outbreaks. In addition, this organism has been reported to cause pneumonia and urinary tract infections.15,16
As mentioned above, for the purpose of this study, bacteremia was defined as two blood culture sets testing positive or one blood culture set testing positive plus a positive culture result for a specimen from another site. There is, however, evidence that bacterial skin commensals isolated from even one blood culture specimen might reflect clinically relevant infection.17,18 Several studies have reported that the time to positivity can be used to discriminate between contamination and bacteremia.19–21 Zhang et al.21 reported that the time to positivity was <36h in 98% of cases of bacteremia caused by Gram-positive bacteria. In our study, although the exact time to positivity (in hours) was not recorded, the time to positivity (in days) was significantly longer in the contamination group and was within three days in all cases of bacteremia. In the present study, a time to positivity of four days or more suggested contamination.
Past studies have generally reported on the susceptibility of C. striatum to the β-lactam group of antimicrobials.1,22,23 However, with increasing use of broad-spectrum antibiotics, recent studies have shown the emergence of multidrug resistant strains.24,25 In our study, most isolated strains were multidrug resistant, and all tested strains were susceptible only to glycopeptides, such as vancomycin and teicoplanin. Based on our results (and in accordance with those of previous studies), when infection with Corynebacterium spp. is suspected, initial therapy should include vancomycin because in vitro resistance to vancomycin has not been reported.2,25
All but one patient in this study received prompt treatment with an antimicrobial agent to which the isolated Corynebacterium spp. was susceptible. Nonetheless, three patients died; their comorbidities were malignancy (n=2) and immunosuppression (n=1). All three patients had central venous catheters in situ; these catheters were not removed because of the severe clinical condition of the patients. Previous studies have reported that while Corynebacterium spp. infection can be associated with death, most cases are relatively easy to manage with antimicrobial therapy and catheter removal, if applicable.26–28 Kimura et al.29 also reported that catheter removal within a week tended to be associated with better outcomes in patients with a central venous catheter.
This study has some limitations. First, it was a retrospective study. Second, we could not clearly distinguish between the true infection group and contamination group because of the absence of established clinical and bacteriological markers of infection with Corynebacterium spp.18,30 Therefore, some patients with contamination may have been included in the bacteremia group, and vice versa. However, all patients in the bacteremia group were considered to have bacteremia and all received treatment for bloodstream infection with Corynebacterium spp., based on their clinical course, culture results, and the recommendation of infectious disease specialists. Third, species identification was carried out in only a small number of patients because it is not generally performed in Japan, especially if contamination is strongly suspected. Thus, we could not evaluate the impact of different species on clinical outcomes. Lastly, data on the total duration of therapy were not collected; thus, an important dimension in the treatment and course of bacteremia was neglected.
ConclusionsIn conclusion, our analysis showed that Corynebacterium spp. isolated from blood culture specimens can cause bloodstream infections in a considerable proportion of patients. Younger age, shorter time to culture positivity, and the presence of an indwelling catheter are associated with bacteremia. Because multidrug-resistant Corynebacterium spp. have become common, administration of appropriate antimicrobial agents, such as vancomycin, should be initiated pending the results of antimicrobial susceptibility testing in patients with suspected bacteremia caused by Corynebacterium spp.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interestThe authors declare no conflicts of interest.
We would like to thank Elsevier (http://webshop.elsevier.com/languageservices/) for English language editing.