The in vitro susceptibility of 105 clinical and environmental strains of Aspergillus fumigatus and Aspergillus flavus to antifungal drugs, such as amphotericin B, azoles, and echinocandins was evaluated by the broth microdilution method proposed by the European Committee on Antimicrobial Susceptibility Testing (EUCAST). Following the EUCAST-proposed breakpoints, 20% and 25% of the clinical and environmental isolates of A. fumigatus, respectively, were found to be resistant to itraconazole (Minimal Inhibitory Concentration, MIC>2.0mg/L). Voriconazole showed good activity against A. fumigatus and A. flavus strains, except for one clinical strain of A. fumigatus whose MIC was 4.0mg/L. Posaconazole (≤0.25mg/L) also showed appreciable activity against both species of Aspergillus, except for six A. fumigatus strains with relatively higher MICs (0.5mg/L). The MICs for Amphotericin B ranged from 0.06 to 1.0mg/L for A. fumigatus, but were much higher (0.5–8.0mg/L) for A. flavus. Among the echinocandins, caspofungin showed a geometric mean of 0.078 and 0.113 against the clinical and environmental strains of A. flavus, respectively, but had elevated minimal effective concentrations (MECs) for seven of the A. fumigatus strains. Anidulafungin and micafungin exhibited considerable activity against both A. fumigatus and A. flavus isolates, except for one environmental isolate of A. fumigatus that showed an MEC of 1mg/L to micafungin. Our study proposes that a detailed investigation of the antifungal susceptibility of the genus Aspergillus from different regions of Brazil is necessary for establishing a response profile against the different classes of antifungal agents used in the treatment of aspergillosis.
Aspergillosis includes a wide range of diseases caused by the filamentous fungus Aspergillus that affects mainly the respiratory tract of immunocompromised patients. It is characterized by invasive and chronic pulmonary infection, allergic reaction, or fungal growth. Aspergillus fumigatus, followed by Aspergillus flavus, are the most common species causing aspergillosis.1,2 The treatment of these diseases is based on the use of azole antifungal drugs, such as voriconazole (VCZ), which is the treatment of choice, itraconazole (ITZ), posaconazole (PCZ), and more recently, isavuconazole (ISZ).3,4 Nevertheless, many studies have reported resistance of A. fumigatus to the azole antifungal drugs that is often due to the cross-resistance to the agricultural triazoles. Resistance rates vary widely across medical centers around the world, with some studies showing high resistance rates5–7 and others with rates even lower than 1%.8,9 As an alternative to the use of azoles, lipid formulations of amphotericin B (AMB) and echinocandins are also being used nowadays in the treatment of aspergillosis.3,4
Owing to the widespread use of azoles in the treatment of aspergillosis and the potential consequence of acquiring azole resistance, antifungal susceptibility studies of the concerned fungal species have become increasingly important in order to understand their resistance profile in each medical center and improve local empirical treatment. In this study, we evaluated the susceptibility profile of A. fumigatus and A. flavus, isolated from patients with different forms of aspergillosis and from the environment in southern Brazil, against azoles, AMB, and echinocandins by using the broth microdilution methodology.
Materials and methodsAspergillus strainsTwenty-five clinical strains of A. fumigatus and 20 of A. flavus were isolated from patients with sinusitis, invasive or cutaneous aspergillosis; 20 strains of A. fumigatus and 40 of A. flavus were isolated from maize crops belonging to the Laboratory of Mycological Research of the Federal University of Santa Maria in southern Brazil. Analyses of macro and micromorphology, growth of A. fumigatus at 48°C, and sequencing of the internal transcribed spacer (ITS 1 and 2) regions were performed to identify the species. ITS region amplifications were performed via PCR using ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTAT TGATATG-3′) primers, as described previously.10 The strains were stored in 10% glycerol at −80°C and were subcultured on Potato Dextrose Agar (PDA) at 27°C for three days or until sporulation. In all the tests, A. fumigatus ATCC 204305 and A. flavus ATCC 204304 strains were used as the internal quality controls.
Antifungal susceptibility testingThe MICs of ITZ, PCZ, VCZ (Sigma–Aldrich, São Paulo, Brazil), and AMB (Leadiant Biosciences, Maryland, USA) and the MECs of the echinocandins such as anidulafungin (AFG; Pharmacia & Upjohn Co. Kalamazoo, MI, USA), caspofungin (CAS; Laboratories Merck Sharp & Dohme-Chibret, Clermont-Ferrand, France), and micafungin (MFG; Astellas Pharma Tech Co., Takaoka, Toyama, Japan) were determined following the recommendations of the EUCAST protocol for filamentous fungi (EUCAST, 2008). All antifungal drugs were solubilized in dimethyl sulfoxide (DMSO) and the working solutions were prepared in RPMI 1640 medium with l-glutamine (Sigma–Aldrich, São Paulo, SP, Brazil). The final drug concentrations ranged from 0.063 to 32.0mg/L for ITZ and VCZ, 0.008 to 4.00mg/L for PCZ, 0.016 to 8.00 for AMB, and 0.0005 to 1.00mg/L for the echinocandins.
Conidial suspensions were obtained from sporulated Aspergillus cultures and adjusted to contain 2–5×106conidia/mL by counting in a hemocytometer. To obtain a final concentration of 2–5×105conidia/mL, 1:10 dilutions were prepared in sterile distilled water. For the microplate preparation, in each well, 100μL of the final conidial suspension were added to 100μL of each of the antifungal drug concentrations. Growth and negative control are included in all tests. The microplates were incubated at 35°C. The MEC readings for echinocandins, defined as the lowest concentration leading to the growth of the abnormal, branched, and short hyphae as compared to those forming long and unbranched hyphae in the growth control, were performed 24h post-incubation. The MIC readings for azoles and AMB, defined as the lowest concentration that completely inhibits the growth compared to that obtained in case of the control, were taken 48h post-incubation.
Data analysesGeometric mean (GM), MIC/MEC50 (minimal inhibitory/effective concentration that inhibits the growth of 50% of the strains) and MIC/MEC90 (minimal inhibitory/effective concentration that inhibits the growth of 90% of the strains) values were calculated by Microsoft Office Excel 2016 (Microsoft Informatica Ltda., São Paulo, Brazil). In order to define the susceptibility of A. fumigatus and A. flavus to azole antifungal agents and AMB, the EUCAST breakpoints were used.11,12 The results with AMB were also analyzed according to the breakpoints suggested by Elefanti et al.13 and CLSI (M38-A2, 2008) method.14 For echinocandins, the CAS ECVs (epidemiological cut-off values) proposed by Espinel-Ingroff et al.15 were used in the interpretation of the results.
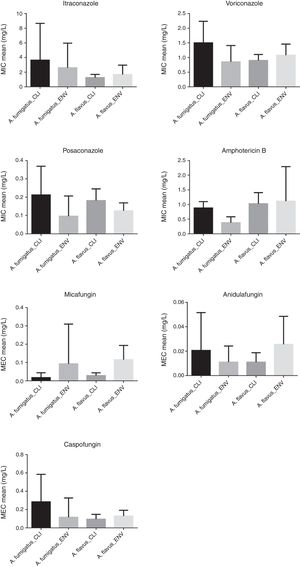
ResultsThe in vitro activities (GM, MIC/MEC50, MIC/MEC90 and MIC/MEC range) of each antifungal drug against the A. fumigatus and A. flavus clinical and environmental strains are presented in the Table 1 and Fig. 1. Fig. 2 shows the percentages of susceptibility, intermediate profile, and resistance against azoles and AMB considering the breakpoints as described above.
In vitro susceptibility of A. fumigatus and A. flavus clinical and environmental isolates against azoles, echinocandins and amphotericin B.
| Drugs | Minimal inhibitory concentration (MIC)/minimal effective concentration (MEC) (mg/L) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. fumigatus (clinical isolates; n=25) | A. fumigatus (environmental isolates; n=20) | A. flavus (clinical isolates; n=20) | A. flavus (environmental isolates; n=40) | |||||||||||||
| Range | GM | 50% | 90% | Range | GM | 50% | 90% | Range | GM | 50% | 90% | Range | GM | 50% | 90% | |
| ITZ | 1.00–16.00 | 2.00 | 1.00 | 16.00 | 1.00–16.00 | 1.803 | 1.00 | 4.00 | 1.00–2.00 | 1.189 | 1.00 | 2.00 | 0.50–8.00 | 1.414 | 1.00 | 2.00 |
| VCZ | 0.50–4.00 | 1.357 | 1.00 | 2.00 | 0.25–2.00 | 0.707 | 0.50 | 2.00 | 0.50–1.00 | 0.871 | 1.00 | 1.00 | 0.50–2.00 | 1.017 | 1.00 | 2.00 |
| PCZ | 0.06–0.50 | 0.169 | 0.125 | 0.50 | 0.03–0.50 | 0.065 | 0.06 | 0.125 | 0.125–0.25 | 0.171 | 0.125 | 0.25 | 0.03–0.25 | 0.188 | 0.125 | 0.125 |
| AMB | 0.50–1.00 | 0.847 | 1.00 | 1.00 | 0.06–1.00 | 0.329 | 0.25 | 0.50 | 0.50–2.00 | 0.966 | 1.00 | 1.00 | 0.50–8.00 | 0.917 | 1.00 | 1.00 |
| MFG | 0.001–0.125 | 0.007 | 0.008 | 0.031 | 0.002–1.00 | 0.031 | 0.03 | 0.125 | 0.008–0.006 | 0.024 | 0.03 | 0.06 | 0.004–0.25 | 0.085 | 0.125 | 0.25 |
| AFG | 0.001–0.125 | 0.007 | 0.004 | 0.063 | 0.001 to −0.006 | 0.007 | 0.008 | 0.015 | 0.002–0.03 | 0.009 | 0.008 | 0.016 | 0.002–0.125 | 0.019 | 0.016 | 0.06 |
| CAS | 0.06–1.00 | 0.188 | 0.125 | 1.00 | 0.03–1.00 | 0.070 | 0.06 | 0.125 | 0.008–0.250 | 0.078 | 0.06 | 0.125 | 0.03–0.25 | 0.113 | 0.125 | 0.25 |
ITZ, itraconazole; VCZ, voriconazole; PCZ, posaconazole; AMB, amphotericin B; MFG, micafungin; AFG, anidulafungin; CAS, caspofungin; GM, geometric mean; 50%, minimal inhibitory concentration/minimal effective concentration that inhibited the growth of 50% of the isolates; 90%, minimal inhibitory concentration/minimal effective concentration that inhibited the growth of 90% of the isolates.
Against ITZ the clinical and environmental isolates of A. fumigatus showed susceptible profile for 52% and 50% of the strains, respectively. Resistance (MIC≥4.0mg/L) was observed in 20% of clinical strains and 25% of environmental strains and intermediate profile (MIC=2.0mg/L) was observed in 28% of clinical strains and 25% of environmental strains. A. flavus isolates showed a good susceptibility profile to ITZ, with only 7.5% (three strains) of environmental isolates being resistant (MIC≥4.0mg/L).
VCZ and PCZ showed to be effective against the clinical and environmental isolates of A. fumigatus and A. flavus. Except for one clinical isolate that showed MIC=4.0mg/L for VCZ, resistance was not observed in any other Aspergillus isolate against this antifungal. Five clinical isolates and one environmental isolate of A. fumigatus showed resistant profile (MIC=0.5mg/L) to PCZ whereas 100% of both types of isolates of A. flavus had a susceptible profile.
AMB MICs for A. fumigatus ranged from 0.5 to 1.0mg/L (clinical isolates) and 0.06 to 1.0mg/L (environmental isolates), whereas for A. flavus the MICs ranged from 0.5 to 2.0mg/L to clinical isolates and 0.5 to 8.0mg/L to environmental isolates.
Echinocandins showed good activity against both Aspergillus species, with GMs of 0.024 for MFG, 0.009 for AFG, and 0.078 for CAS in clinical isolates and 0.085 for MFG, 0.019 for ANF and 0.113 for CAS in environmental isolates of A. flavus. For A. fumigatus MEC=1.0mg/L of CAS was observed against three clinical isolates (GM=0.188) and one environmental isolate (GM=0.070). In addition one environmental isolate also had MIC=1.0mg/L for MFG. The ANF MECs ranged from 0.001 to 0.125mg/L in clinical isolates and 0.001 to 0.06mg/L in environmental isolates.
DiscussionThe in vitro antifungal susceptibility of A. fumigatus and A. flavus, recovered from patients and from environmental sources in Brazil, to azoles, AMB, and echinocandins have been described in this study. In general, all the antifungal drugs showed appreciable activity against both species. However, A. fumigatus strains were found to be less susceptible to ITZ, while A. flavus strains were less susceptible to AMB.
Several studies with these fungal strains have reported resistance to azole antifungals, especially ITZ, with high rates reported in European countries, such as the Netherlands and United Kingdom, where azole resistance rates reached 38%.16,17 This rate of resistance has been attributed to the long-term azole therapy in patients with chronic aspergillosis, in addition to cross-resistance with agricultural triazoles.5,18
In Brazil, epidemiological data on the susceptibility of Aspergillus isolates recovered from both patients as well as from the environment are quite scarce. Although resistance is observed in patients treated with azoles, susceptibility testing is not common in routine laboratory tests. In the current study, we demonstrated 20–25% rate of ITZ resistance in clinical and environmental isolates of A. fumigatus, which is alarming considering the facts that triazoles are the first line of treatment for aspergillosis and that azole fungicides are heavily used in Brazilian agriculture.19
Resistance to triazoles have already been described in many other Latin American countries. In Colombia, 19 environmental strains of A. fumigatus, collected from flower fields and other sites in the city of Bogota, were reported to be azole-resistant and contained alterations in the Cyp51A gene, mainly 46bp tandem repeat combined with Y121F and T289A point mutations (TR46/Y121F/T289A).20 In Argentina, an A. fumigatus strain, recovered from a patient with keratitis, has also been shown resistant to ITZ, and harbored the G54E mutation in Cyp51A.21 In contrast, a multicenter international surveillance network performed by van der Linden et al.22 tested 3788 Aspergillus strains from different countries for azole resistance, among which 64 clinical strains recovered from Brazilian patients showed no resistance to ITZ. In addition, Negri et al.23 recently reported non-resistance to ITZ and PSC, and only intermediate MICs (2.0mg/L) for few strains to VCZ in a study with 221 Brazilian clinical strains of A. fumigatus.
VCZ and PCZ were also found to have good activity against A. fumigatus strains. While VCZ has been used in the clinic recently, PCZ is yet to be licensed for the treatment of patients in Brazil. Although five clinical strains of A. fumigatus had MIC=0.5mg/L of PCZ, which is just above the breakpoint concentration, we believe that a definitive conclusion about this azole antifungal susceptibility will not be reached before PCZ is clinically accepted in the Brazilian medical centers. Only one clinical strain of A. fumigatus showed an MIC of 4.0mg/L for VCZ, which shows that this azole is considered a good treatment choice. However, adequate use should always be taken into account for this profile to be maintained. In contrast to our results, a study by van Ingen et al.17 in the Netherlands showed that when resistant to ITZ, approximately 92–97% of A. fumigatus strains were also resistant to VCZ and PCZ. The higher use of VCZ and PCZ in medical centers around the world may explain this contrast.
On the other hand, all azole antifungals showed considerably good activity against A. flavus, except three strains of environmental origin that were resistant to ITZ (MIC=4.0; n=2 and 8.0mg/L; n=1). Many supportive studies have shown A. flavus to be quite susceptible to azole antifungals. Shivaprakash et al.24 tested 188 clinical and environmental isolates of A. flavus from India and the Netherlands, all of which were susceptible to the triazoles tested, except three strains that had MICs of 4.0mg/L. In Brazil, Gonçalves et al.25 also showed good response of A. flavus to azole antifungals, with MIC ranging from 0.25 to 2.0mg/L.
Following the EUCAST26 breakpoints, both isolates of A. fumigatus and A. flavus were found to be 100% susceptible to AMB. However, considering the CLSI breakpoints,14 76% of clinical A. fumigatus showed an intermediate profile while 10% of the clinical and environmental isolates of A. flavus showed a resistant profile (MIC of AMB ≥2.0mg/L). If we base our analyses on the breakpoints proposed by Elefanti et al. in 2014,13 the same susceptibility profile is obtained for A. fumigatus as that obtained from CLSI breakpoints; however, 85% of the clinical and 72.5% of the environmental A. flavus isolates showed a resistant profile with MIC of AMB ≥1.0mg/L.
Although the interpretations from different proposed breakpoints may differ, AMB was seen to have lesser activity against A. flavus than against A. fumigatus. This could be due to mutations in the ergosterol biosynthesis pathway that lead to a decrease in ergosterol concentration in the fungal cellular membrane, thereby decreasing the targets of AMB action in A. flavus.27 Our results are in agreement with those of other studies that evaluated the susceptibility profile to AMB of A. flavus. Gonçalves et al.25 reported 49% resistance to AMB (MIC ≥2.0mg/L) in 77 clinical isolates belonging to the section Flavi. Sabatelli et al.28 also demonstrated higher activity of AMB against A. fumigatus (MIC90=1.0mg/L; n=1.119) than that against A. flavus (MIC90=2.0mg/L; n=89) isolates. More recently, Taghizadeh-Armaki et al.29 also showed reduced susceptibility of environmental and clinical strains of A. flavus to AMB.
Of the three echinocandins tested here, only CAS and MFG showed increased MECs (≥0.5mg/L) against six and one clinical isolates of A. fumigatus, respectively. The MECs of CAS against A. fumigatus were lower for environmental strains than for the clinical ones, whereas for A. flavus, the mean MECs showed the opposite trend in the two strains.
ANF and MFG showed lower mean MECs compared to CAS for both A. fumigatus and A. flavus strains. AFG was found capable of causing alterations in hyphae at concentrations lower than those of MFG for all the isolates, except for A. fumigatus clinical strains in which the same mean MEC was observed for both MFG and AFG. Other authors have reported better activity of ANF against Aspergillus spp.30,31 compared to the other echinocandins.
CAS is successfully used in salvage therapy for invasive aspergillosis, while the two other echinocandins mentioned here have not yet been recommended clinically owing to the lack of reliable and effective dose definitions. Echinocandin resistance is not common in Aspergillus; however, a few reports of CAS resistance have emerged.31 Although it has not been possible to establish a reliable relationship between the MEC and clinical response, based on the ECVs reported by Elefanti et al.,15 we observed a lower action of CAS in some of the clinical A. fumigatus strains studied here, hinting at the possibility of resistance development during treatment.
Information regarding the antifungal susceptibility of Aspergillus in Brazil and other Latin American countries is still scarce because of the lack of routine susceptibility tests in most of the clinical laboratories. Although the current study could not evaluate a large number of strains, it could be clearly concluded that a significant percentage of the clinical and environmental strains of A. fumigatus were azole-resistant, whereas A. flavus especially showed less susceptibility to AMB, and echinocandins presented elevated MECs against some strains. Larger studies with strains from different regions of Brazil are urgently needed, not only to increase the awareness about the antifungal susceptibility of this important fungal pathogen in our country, but also to implement a better management of aspergillosis in our medical centers, thereby reducing the resistance rates.
Conflicts of interestThe authors declare no conflicts of interest.
This study was supported by the Higher Education Personnel Training Coordination (CAPES), Brazil. Alves SH thanks the financial support provided by the Brazilian Agencies FAPERGS (Grant Proc. 2261-12) and CNPq (Grant Proc. 302797/2016-5).