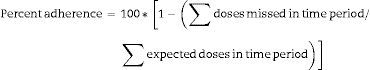Few studies have examined antiretroviral therapy adherence in Latin American children. Standardized behavioral measures were applied to a large cohort of human immunodeficiency virus-infected children in Brazil, Mexico, and Peru to assess adherence to prescribed antiretroviral therapy doses during the three days prior to study visits, assess timing of last missed dose, and evaluate the ability of the adherence measures to predict viral suppression. Time trends in adherence were modeled using a generalized estimating equations approach to account for possible correlations in outcomes measured repeatedly in the same participants. Associations of adherence with human immunodeficiency virus viral load were examined using linear regression. Mean enrollment age of the 380 participants was 5 years; 57.6% had undetectable’ viral load (<400copies/mL). At enrollment, 90.8% of participants were perfectly (100%) adherent, compared to 87.6% at the 6-month and 92.0% at the 12-month visit; the proportion with perfect adherence did not differ over time (p=0.1). Perfect adherence was associated with a higher probability of undetectable viral load at the 12-month visit (odds ratio=4.1, 95% confidence interval: 1.8–9.1; p<0.001), but not at enrollment or the 6-month visit (p>0.3). Last time missed any antiretroviral therapy dose was reported as “never” for 52.0% at enrollment, increasing to 60.7% and 65.9% at the 6- and 12-month visits, respectively (p<0.001 for test of trend). The proportion with undetectable viral load was higher among those who never missed a dose at enrollment and the 12-month visit (p≤0.005), but not at the 6-month visit (p=0.2). While antiretroviral therapy adherence measures utilized in this study showed some association with viral load for these Latin American children, they may not be adequate for reliably identifying non-adherence and consequently children at risk for viral resistance. Other strategies are needed to improve the evaluation of adherence in this population.
In 2012, there were approximately 56,000 children under 15 years of age living with HIV in Latin America and the Caribbean.1 With increased availability of antiretroviral therapy (ART), there has been a significant decrease in the morbidity and mortality of perinatally infected children.2,3 Success in achieving good outcomes relies on high levels of ART adherence to maximize clinical effectiveness and limit potential for development of drug resistance.4
Few studies from Latin America have estimated ART adherence levels in children or evaluated the validity of methods used to measure adherence.5 Among the various methods used in resource-limited settings, behavioral measures of adherence, including self- and caregiver-report, are the most common.6 Behavioral methods are attractive because they are practical and inexpensive. However, few studies in resource-limited settings have attempted to validate their accuracy by comparing them with other adherence measurement tools, and those that have produced mixed results.7–12 In this sub-study of the NICHD (Eunice Kennedy Shriver National Institute of Child Health and Human Development) International Site Development Initiative (NISDI) PLACES (Pediatric Latin American Countries Epidemiologic Study) protocol, we assessed ART adherence levels and evaluated the ability of the adherence measures to predict viral suppression among children living with HIV in Latin America.
Materials and methodsParticipantsParticipants were children living with HIV and their caregivers that enrolled in PLACES, a prospective cohort study that enrolled perinatally HIV-infected children less than 6 years of age at the time of enrollment at 14 clinical sites (12 in Brazil, 1 each in Peru and Mexico). The protocol was approved by the ethical review boards of each clinical site, the sponsoring institution (NICHD), the data management and statistical center (Westat), and the Brazilian National Ethics Committee (CONEP). Informed consent was obtained from the parents or guardians prior to enrollment.
A description of the earlier version of the protocol and the cohort, including the site selection process, has been published elsewhere.13 In brief, demographic, laboratory, and clinical data were collected at enrollment and every 6 months, including HIV-1 RNA viral load (VL), CD4 measures, CDC classification, and antiretroviral medication adherence.
Adherence measuresART adherence was assessed through a structured questionnaire developed for use by the U.S. National Institute of Allergy and Infectious Diseases (NIAID) as part of standard practice in PACTG (Pediatric AIDS Clinical Trials Group) studies.14 The potential for social desirability bias with self-/caregiver-reported adherence was considered in the design of the PACTG instrument and the instructions for its administration, which were followed in our study. These instructions emphasize that the accuracy of self-report is very good if the attitude of the interviewer is non-judgmental and supportive. To set the proper tone, the adherence form includes introductory statements acknowledging how difficult adherence can be that were read verbatim. The participant/caregiver was asked to identify the ARV medications and number of doses (not number of pills) prescribed each day. The participant/caregiver was prompted regarding any omitted medications if all of the prescribed ARV medications identified during medical chart review by the interviewer were not reported. Interviewees were then asked to report the number of missed doses for each ARV medication for each of the previous three days. The interviewer asked about specific problems that may have been encountered in giving or taking medications. Instructions printed on the form stressed that any interaction occurring after the form was completed in response to non-adherence was critically important, noting that the attitude of the interviewer in response to non-adherence, the manner in which adherence would be promoted, and the nature of any behavioral counseling offered would absolutely influence the validity of subsequent self-report data.
The interview was administered in Spanish or Portuguese by a member of the clinical care or research team to the person with primary responsibility for medication administration. Certified translations were performed by an independent company using language experts (English and the relevant language for translation) that had a scientific background and were well versed in medical terminology.
ART adherence was derived based on the total number of doses missed during the three-day period prior to a study visit and the total number of expected doses for all of the ARVs included in the participant's treatment regimen at the time of the visit. The measure was expressed in the form of a continuous measure of percent adherence calculated as shown below; adherence was also examined on the basis of a binary indicator of perfect (100%) adherence.
Participants/caregivers were also asked to recall when they/the child last missed a dose of any ARV medication; response options included never, during the previous two weeks, during the last month, over a month ago or don’t remember. This measure was dichotomized for purposes of analysis (never vs. ever).
Statistical analysesSimple descriptive statistics (mean, standard deviation, median, frequency count, percentage) were used to describe characteristics of the study population and adherence measures. Trends in adherence and VL across the three study visits (enrollment and 6 and 12 months post-enrollment) were modeled using a generalized estimating equations (GEE) approach to account for possible correlations in outcomes when measured repeatedly in the same participants. The association of behavioral measures of ART adherence with VL, a standard biomarker of adherence, was examined using linear regression modeling and Fisher's exact test. VL values below the limit of detection were set to one-half of the lower limit of detection for the assay for purposes of analysis (i.e., VL <400 set to 200), while those reported as greater than a specified value were set to the associated value (i.e., VL >500,000 set to 500,000). All analyses were performed using the SAS software system, version 9.2, with an alpha level of 0.05 used in assessing statistical significance using two-tailed tests.
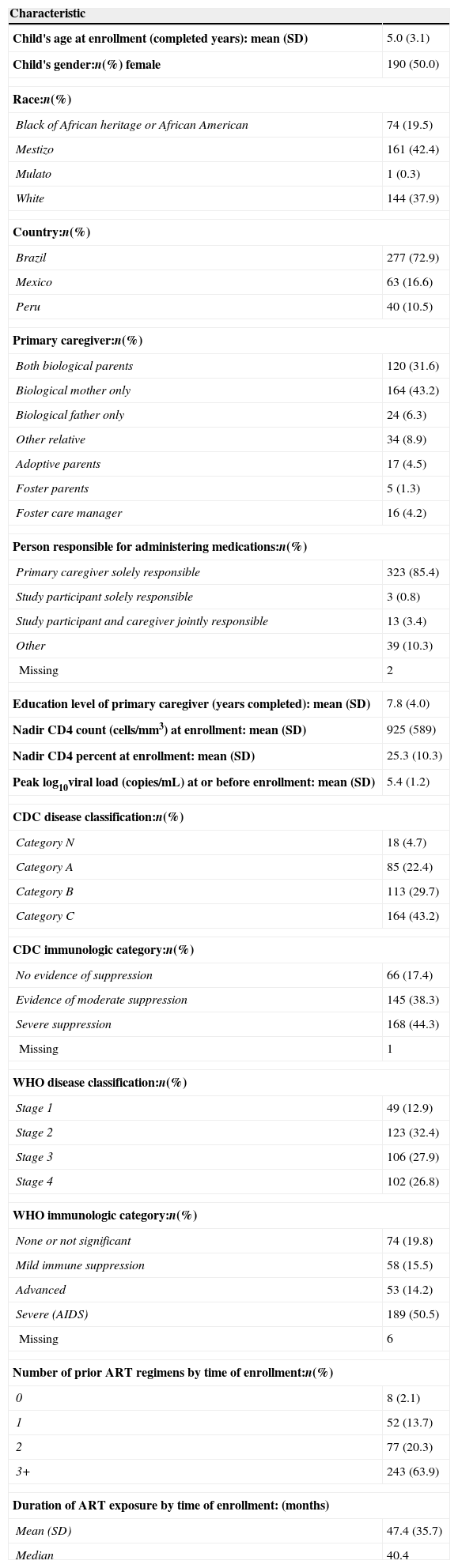
ResultsAmong the 500 PLACES study participants, there were 387 who were currently prescribed HIV medications at the time of enrollment and therefore eligible for inclusion in the analyses; the 113 participants not currently prescribed ART at enrollment were excluded from the analysis. An additional seven participants that had assumed responsibility for their own drug regimen and completed the adherence interview themselves were excluded from the analysis to create a more homogenous population for investigation. Characteristics of the 380 participants included in the analyses are described in Table 1. The mean age of the participants was 5.0, ranging from <1 to 11 years, and most were enrolled in Brazil (72.9%). The primary caregiver was a biological parent for 81.1% of children. A primary caregiver had sole responsibility for administering ARTs for 85.4% of children, while only 0.8% of study participants assumed sole responsibility themselves. The mean (±standard deviation) nadir CD4 count at enrollment was 925±589cells/mm3; mean peak log10 viral load (copies/mL) at or before enrollment was 5.4±1.2. Ninety-five percent of the children were on combination ART. Eighty-four percent of study participants had received two or more regimens by the time of study enrollment; the number of ART regimens received considered changes from one drug class to another, but not changes within class. The mean duration of ARV use at enrollment was 47.4±35.7 months.
Characteristics of study population (N=380).
| Characteristic | |
|---|---|
| Child's age at enrollment (completed years): mean (SD) | 5.0 (3.1) |
| Child's gender:n(%) female | 190 (50.0) |
| Race:n(%) | |
| Black of African heritage or African American | 74 (19.5) |
| Mestizo | 161 (42.4) |
| Mulato | 1 (0.3) |
| White | 144 (37.9) |
| Country:n(%) | |
| Brazil | 277 (72.9) |
| Mexico | 63 (16.6) |
| Peru | 40 (10.5) |
| Primary caregiver:n(%) | |
| Both biological parents | 120 (31.6) |
| Biological mother only | 164 (43.2) |
| Biological father only | 24 (6.3) |
| Other relative | 34 (8.9) |
| Adoptive parents | 17 (4.5) |
| Foster parents | 5 (1.3) |
| Foster care manager | 16 (4.2) |
| Person responsible for administering medications:n(%) | |
| Primary caregiver solely responsible | 323 (85.4) |
| Study participant solely responsible | 3 (0.8) |
| Study participant and caregiver jointly responsible | 13 (3.4) |
| Other | 39 (10.3) |
| Missing | 2 |
| Education level of primary caregiver (years completed): mean (SD) | 7.8 (4.0) |
| Nadir CD4 count (cells/mm3) at enrollment: mean (SD) | 925 (589) |
| Nadir CD4 percent at enrollment: mean (SD) | 25.3 (10.3) |
| Peak log10viral load (copies/mL) at or before enrollment: mean (SD) | 5.4 (1.2) |
| CDC disease classification:n(%) | |
| Category N | 18 (4.7) |
| Category A | 85 (22.4) |
| Category B | 113 (29.7) |
| Category C | 164 (43.2) |
| CDC immunologic category:n(%) | |
| No evidence of suppression | 66 (17.4) |
| Evidence of moderate suppression | 145 (38.3) |
| Severe suppression | 168 (44.3) |
| Missing | 1 |
| WHO disease classification:n(%) | |
| Stage 1 | 49 (12.9) |
| Stage 2 | 123 (32.4) |
| Stage 3 | 106 (27.9) |
| Stage 4 | 102 (26.8) |
| WHO immunologic category:n(%) | |
| None or not significant | 74 (19.8) |
| Mild immune suppression | 58 (15.5) |
| Advanced | 53 (14.2) |
| Severe (AIDS) | 189 (50.5) |
| Missing | 6 |
| Number of prior ART regimens by time of enrollment:n(%) | |
| 0 | 8 (2.1) |
| 1 | 52 (13.7) |
| 2 | 77 (20.3) |
| 3+ | 243 (63.9) |
| Duration of ART exposure by time of enrollment: (months) | |
| Mean (SD) | 47.4 (35.7) |
| Median | 40.4 |
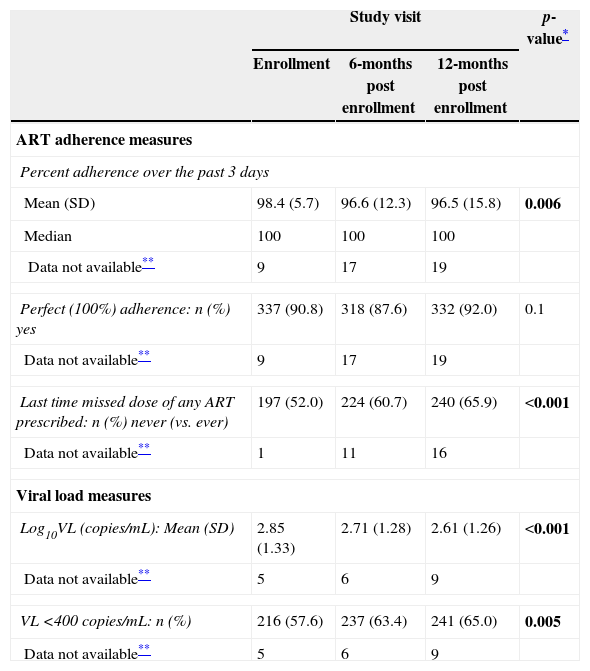
With few exceptions, the expected number of doses per 24-h period abstracted by the study nurse from the medical record equaled the number of doses reported by the primary caregiver for the same period for all study visits (data not shown). Mean (median) percent adherence over the past three days was 98.4% (100%) at enrollment, decreasing modestly to 96.6% (100%) and 96.5% (100%) at the 6- and 12-month visits, respectively (Table 2); 5% or less of study participants did not have adherence data collected/recorded at each study visit. Despite the small magnitude of these differences, a model fit to the data showed that percent adherence varied significantly over time (p=0.006); in pairwise comparisons, percent adherence differed between enrollment and the 6- and 12-month visits (p<0.04), but not between the 6- and 12-month visits (p=1.0). At enrollment, 90.8% of participants were perfectly (100%) adherent, compared to 87.6% at the 6-month and 92.0% at the 12-month visit; the proportion with perfect adherence did not differ over time (p=0.1). When doses were missed, they were typically missed only for a subset of the drugs included within the prescribed regimen, they were usually missed for only one of the three days targeted by the adherence questionnaire, and the ART doses that were missed tended to represent only a portion of the expected number of doses rather than all doses of a specific medication for a given day (data not shown).
Description of behavioral measures of ART adherence and viral load measures by study visit (n=380).
| Study visit | p-value* | |||
|---|---|---|---|---|
| Enrollment | 6-months post enrollment | 12-months post enrollment | ||
| ART adherence measures | ||||
| Percent adherence over the past 3 days | ||||
| Mean (SD) | 98.4 (5.7) | 96.6 (12.3) | 96.5 (15.8) | 0.006 |
| Median | 100 | 100 | 100 | |
| Data not available** | 9 | 17 | 19 | |
| Perfect (100%) adherence: n (%) yes | 337 (90.8) | 318 (87.6) | 332 (92.0) | 0.1 |
| Data not available** | 9 | 17 | 19 | |
| Last time missed dose of any ART prescribed: n (%) never (vs. ever) | 197 (52.0) | 224 (60.7) | 240 (65.9) | <0.001 |
| Data not available** | 1 | 11 | 16 | |
| Viral load measures | ||||
| Log10VL (copies/mL): Mean (SD) | 2.85 (1.33) | 2.71 (1.28) | 2.61 (1.26) | <0.001 |
| Data not available** | 5 | 6 | 9 | |
| VL <400copies/mL: n (%) | 216 (57.6) | 237 (63.4) | 241 (65.0) | 0.005 |
| Data not available** | 5 | 6 | 9 | |
p-values <0.05 are bolded.
When asked about the last time a dose of any prescribed ART was missed, a large proportion of participants at each visit never missed a dose (52.0% at enrollment, 60.7% at 6 months, 65.9% at 12 months) (Table 2); this measure of adherence varied significantly over time (p<0.001). The probability of never missing a dose was higher at the 6- and 12-month visits, compared to the enrollment visit (odds ratio (OR)=1.4 [95% confidence interval (CI): 1.1–1.8; p=0.003] and 1.8 [95% CI: 1.4–2.3; p<0.001], respectively), but did not differ between the 6- and 12-month visits (p=0.07).
Mean (±SD) log10 VL varied over time (p<0.001) (Table 2), with pairwise comparisons indicating that VL at the 6- and 12-month visits was significantly lower than at enrollment (p=0.01 and <0.001, respectively), while the difference between the 6- and 12-month visits was of marginal significance (p=0.052). Similarly, the occurrence of VL <400copies/mL varied significantly over time (p=0.005), with 57.6% at enrollment, compared to 63.4% at the 6-month and 65.0% at the 12-month visits. The probability of having a VL measure <400copies/mL at 6 (OR=1.3, 95% CI: 1.1–1.5; p=0.01) and 12 months (OR=1.4, 95% CI: 1.1–1.7; p=0.002) was higher than at the enrollment visit, but did not differ between the 6- and 12-month visits (p=0.4).
Regression modeling indicated that percent adherence was inversely, but not significantly, associated with log10 VL at the enrollment or 6-month visit (p>0.1) (data not shown). At 12 months, a 5-percent increase in adherence was associated with a small (−0.09) decline in log10 VL (p<0.001); the model indicated that only 5.7% of the variation in VL could be explained by variability in percent adherence.
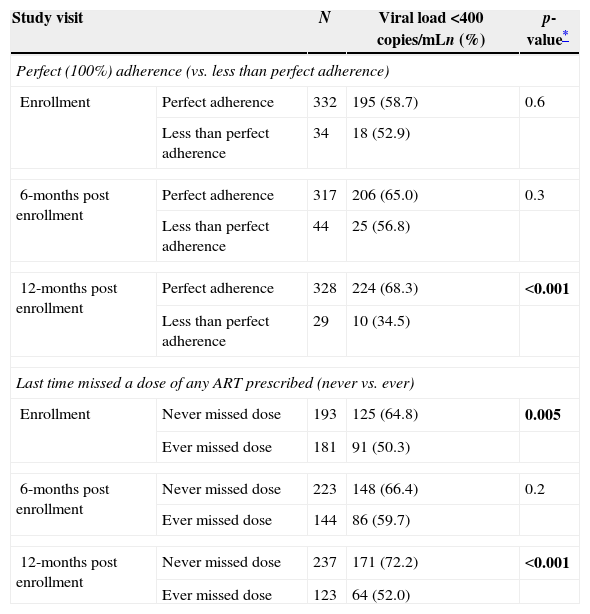
Perfect adherence was associated with VL <400copies/mL only at the 12-month study visit (p<0.001) (Table 3); a higher proportion of those with perfect adherence had VL <400copies/mL (68.3%) than among those with less than perfect adherence (34.5%). The probability of having a VL measure <400copies/mL at 12 months was more than four times higher among those with perfect adherence than those with less than perfect adherence (OR=4.1, 95% CI: 1.8–9.1).
Association of perfect adherence and long-term behavioral adherence measure with viral load <400copies/mL at enrollment and the 6- and 12-month post enrollment visits.
| Study visit | N | Viral load <400copies/mLn (%) | p-value* | |
|---|---|---|---|---|
| Perfect (100%) adherence (vs. less than perfect adherence) | ||||
| Enrollment | Perfect adherence | 332 | 195 (58.7) | 0.6 |
| Less than perfect adherence | 34 | 18 (52.9) | ||
| 6-months post enrollment | Perfect adherence | 317 | 206 (65.0) | 0.3 |
| Less than perfect adherence | 44 | 25 (56.8) | ||
| 12-months post enrollment | Perfect adherence | 328 | 224 (68.3) | <0.001 |
| Less than perfect adherence | 29 | 10 (34.5) | ||
| Last time missed a dose of any ART prescribed (never vs. ever) | ||||
| Enrollment | Never missed dose | 193 | 125 (64.8) | 0.005 |
| Ever missed dose | 181 | 91 (50.3) | ||
| 6-months post enrollment | Never missed dose | 223 | 148 (66.4) | 0.2 |
| Ever missed dose | 144 | 86 (59.7) | ||
| 12-months post enrollment | Never missed dose | 237 | 171 (72.2) | <0.001 |
| Ever missed dose | 123 | 64 (52.0) | ||
p-values <0.05 are bolded.
The proportion of participants with VL <400copies/mL at enrollment was higher among those who reported never missing any medication dose vs. those that had missed a dose (64.8% vs. 50.3%, p=0.005) (Table 3). Likewise, at the 6-month visit, subjects who never missed a dose were more likely to have viral load <400copies/mL (66.4%) than those who did miss a dose (59.7%), but this difference was not statistically significant (p=0.2). At the 12-month visit, the difference was again statistically significant (p<0.001), as the proportion with VL <400copies/mL at 12 months was higher among those who reported never missing an ART medication dose (72.2%) compared to those who had (52.0%).
DiscussionThe mean caregiver reported ART adherence was above 96% at each targeted study visit for this cohort of young children living with HIV in Latin America. The majority of study participants did not report missing any doses of their prescribed ART regimen in the past three days. Those that did report missing doses typically missed only some of the doses for some of the drugs included within their prescribed regimen, and usually missed doses for only one of the three days assessed.
Despite their widespread use, research is mixed on the utility of self-report adherence measures for children living with HIV. Some previous studies from both developed countries and resource-limited settings have failed to show an association between behavioral measures of ART adherence and VL in HIV-infected pediatric patients, while other studies have found strong associations between VL and behavioral adherence measures.7,12,15–18 The validity of self-reported adherence has been examined in a systematic review of 77 studies19 and in a meta-analysis of 65 studies.20 These reviews concluded that self-report correlates with objective adherence measures including electronic monitoring systems and HIV health indicators, such as VL. Several guidelines have recommended the use of self-report for adherence measurement21,22 since it offers the advantages of low cost, minimal patient and clinician burden, flexible design to suit individual language abilities, and ease of data collection, which are especially useful in resource-limited settings.19,23 Aside from these practical considerations, self-report can facilitate discussion between patients and providers concerning reasons for non-adherence. As previously noted, the adherence instrument used in our study has been validated for use as part of standard practice in PACTG therapy studies.14,24 Beyond its extensive application in U.S. studies, this questionnaire or slight modifications of such have been adopted for use in many countries, including Brazil25; Cambodia26,27; Nepal28; South Africa7; Thailand26,29,30; and Uganda,9 to name a few.
Although neither of our adherence measures were consistently, significantly associated with VL <400copies/mL, consistent trends were observed between VL and perfect adherence, and even more so for the timing of last missed dose of any medication, supporting the use of longer periods of recall for better identifying those with adherence difficulties. One US-based study found a correlation between no doses missed in the previous month and VL, while another study found that, although caregiver 3-day recall and reporting of doses missed during the previous six months were not associated with VL, recall of doses taken over the last 6 months was significantly related to VL.17,31 A meta-analysis examining ART adherence in HIV-infected children, adolescents and young adults that included studies conducted in the US, Africa, Europe, Thailand, Haiti and Brazil, found that the two most frequently assessed time periods for those using self-/caregiver-report were the past month, followed by the past two to four days.32
A major challenge associated with the use of self-report that may have influenced our results is a tendency for overestimation of adherence by participants/caregivers, as this method is susceptible to recall bias and social desirability bias, whereby respondents tend to provide answers to questions that will be viewed favorably by the interviewer.33 A Ugandan study reported adherence to ART of 95% or greater for 89% of children between 2 and 18 years of age using 3-day caregiver/self-report, compared to only 72% for home-based unannounced pill counts, an objective measure of adherence.9 In Cape Town, South Africa, mean adherence according to caregiver-reported visual analog scale (VAS) was 98.6%, while it was only 79.6% according to MEMS (Medication Event Monitoring System).8 While it is true that self-report by children and caregivers may overestimate adherence by about 10–20% compared to electronic monitoring methods,34 other commonly used measures of medication adherence, such as MEMS caps, pill counts, pharmacy records, and blood concentrations, are also subject to limitations that can lead to biased adherence estimates. While unlikely to have eliminated social desirability bias altogether, use of the PACTG adherence questionnaire, with its standardized scripts and method of administration, should have helped in reducing its extent.
Although the NISDI adherence questionnaire had previously been used in developed countries for Spanish speaking individuals, its cultural validity was not tested prior to its use in this Latin American population. This lack of cultural adaptation has been observed for several other pediatric ART adherence questionnaires used in low- and middle-income countries, and may have played a role in limiting the association between reported adherence and VL in this study.6
A major challenge in evaluating the validity of behavioral measures of adherence is the lack of a gold standard for measuring ART adherence in children. Although we assessed the NISDI adherence questionnaire against VL, this is not a perfect benchmark for evaluating how accurately a particular approach measures adherence. Indeed, the relationship between adherence and VL is complex and can be affected by factors other than adherence. Among adults, the risk of virologic failure (≥50copies/mL) was shown to decrease with longer duration of continuous viral suppression, even with adherence of 50–74%.35 In adults receiving NNRTI (Non-nucleoside Reverse Transciptase Inhibitor)-containing regimens, it has been reported that viral suppression can be achieved with adherence levels as low as 54%.36 A study using 3-day report found that the percentage of children with undetectable VL (<400copies/mL) was significantly higher among fully adherent than non-adherent children (50% vs. 27%, p<0.001).24 However, many fully adherent children had detectable VL, possibly due to previous sub-optimal treatment that led to drug resistance and sub-optimal suppression of viral replication, and some non-adherent children had undetectable VL. Recognizing that full adherence would only be expected to lead to undetectable VL for participants receiving effective therapy, the authors examined the relationship of adherence to VL, restricted to those receiving HAART (Highly Active Antiretroviral Therapy) with or without a PI (Protease Inhibitor). The authors found a significant association within this subgroup (p>0.001), but not among those not receiving non-HAART combination regimens or among those receiving ≥3 NRTIs (Nucleoside Reverse Transcriptase Inhibitor).
Achieving correct dosing is particularly challenging in pediatric patients due to limited pediatric fixed dose formulations and differences in the way drugs are metabolized in children, possibly leading to incorrect dosing.37 Consequently a child could have a high level of adherence without viral suppression if his/her regimen is under-dosed. Although a participant might be adherent, their medications may not be taken as directed with respect to the timing of doses and timing of food consumption, which could reduce medication effectiveness.24 Children who have developed resistance would also not be expected to achieve viral suppression despite high levels of adherence; high prevalence of viral resistance has been identified in several cohorts of perinatally infected children.38–40 Finally, children have greater difficulty in achieving optimal viral suppression than adults, which has been attributed to higher baseline viral load and differences in viral dynamics.41
In conclusion, the current ART adherence questionnaire utilized for NISDI pediatric patients demonstrated some association with viral load, but may not be adequate for reliably identifying non-adherence and consequently children at risk for viral resistance. Future studies using behavioral measures of adherence in Latin American populations should consider using longer periods of recall; better framing caregiver and self-report questions; and computer administration of the questionnaires (as opposed to face-to-face interviews) in an effort to reduce social desirability; and working to ensure that the questions are culturally acceptable. The use of more objective measures of adherence in addition to viral load, such as pill counts or pharmacy records, should be considered when possible, as well as combining objective measures with self-report measures.
Financial supportSupported by NICHD Contracts N01-HD-3-3345 (2002–2007), HHSN267200800001C (2007–2012), and HHSN275201300003C (2012–2017).
Conflicts of interestThe authors declare no conflicts of interest.
The NISDI PLACES Study Group
Principal investigators, co-principal investigators, study coordinators, data management center representatives, and NICHD staff include: Brazil:Belo Horizonte: Jorge A. Pinto, Flávia F. Faleiro, Marcelle M. Maia (Universidade Federal de Minas Gerais); Caxias do Sul: Rosa Dea Sperhacke, Nicole Golin, Sílvia Mariani Costamilan (Universidade de Caxias do Sul/Serviço Municipal de Infectologia); Nova Iguacu: Jose Pilotto, Luis Felipe Moreira, Ivete Gomes (Hospital Geral Nova de Iguacu – HIV Family Care Clinic); Porto Alegre: Rosa Dea Sperhacke, Breno Riegel Santos, Rita de Cassia Alves Lira (Universidade de Caxias do Sul/Hospital Conceição); Rosa Dea Sperhacke, Mario Ferreira Peixoto, Elizabete Teles (Universidade de Caxias do Sul/Hospital Fêmina); Rosa Dea Sperhacke, Marcelo Goldani, Carmem Lúcia Oliveira da Silva, Margery Bohrer Zanetello (Universidade de Caxias do Sul/Hospital de Clínicas de Porto Alegre); Regis Kreitchmann, Marcelo Comerlato Scotta, Debora Fernandes Coelho (Irmandade da Santa Casa de Misericordia de Porto Alegre); Ribeirão Preto: Marisa M. Mussi-Pinhata, Maria Célia Cervi, Márcia L. Isaac, Fernanda Tomé Sturzbecher, Bento V. Moura Negrini (Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo); Rio de Janeiro: Ricardo Hugo S. Oliveira, Maria C. Chermont Sapia (Instituto de Puericultura e Pediatria Martagão Gesteira); Esau Custodio Joao, Maria Leticia Cruz, Leon Claude Sidi, Maria Isabel Gouvêa, Mariza Curto Saavedra, Clarisse Bressan, Fernanda Cavalcanti A. Jundi (Hospital dos Servidores do Estado); São Paulo: Regina Celia de Menezes Succi, Daisy Maria Machado (Escola Paulista de Medicina- Universidade Federal de São Paulo); Marinella Della Negra, Wladimir Queiroz, Yu Ching Lian (Instituto de Infectologia Emilio Ribas); Mexico:Mexico City: Noris Pavía-Ruz, Dulce Morales-Pérez, Karla Ojeda-Diezbarroso (Hospital Infantil de México Federico Gómez); Peru:Lima: Jorge O. Alarcón Villaverde (Instituto de Medicina Tropical “Daniel Alcides Carrión”- Sección de Epidemiologia, UNMSM), María Castillo Díaz (Instituto Nacional de Salud del Niño), Mary Felissa Reyes Vega (Instituto de Medicina Tropical “Daniel Alcides Carrión” - Sección de Epidemiologia, UNMSM); Data Management and Statistical Center: Yolanda Bertucci, Laura Freimanis Hance, René Gonin, D. Robert Harris, Roslyn Hennessey, Margot Krauss, Sue Li, Karen Megazzini, Orlando Ortega, James Korelitz, Sharon Sothern de Sanchez, Sonia K. Stoszek, Qilu Yu (Westat, Rockville, MD, USA) NICHD: Rohan Hazra, Lynne M. Mofenson, George K. Siberry (Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Maryland).
We also want to thank the study participants, staff at the clinical sites, and the NISDI Executive Committee.