Despite the high rate of tuberculosis indicators in Brazil, the Federal District shows a low prevalence of the disease.
ObjectiveTo analyze the relationship between climatic factors and air quality with tuberculosis in the Brazilian Federal District.
MethodologyThis was an ecological and descriptive study comparing 3927 new cases of Tuberculosis registered at the Federal District Tuberculosis Control Program with data from the National Institute of Meteorology, Brazilian Institute of Geography and Statistics, Brazilian Agricultural Research Institute, Brasilia Environmental Institute, and the Federal District Planning Company.
ResultsFrom 2003 to 2012, there has been a higher incidence of Tuberculosis (27.0%) in male patients in the winter (27.2%). Patients under 15 years of age (28.6%) and older than 64 years (27.1%) were more affected in the fall. For youth and adults (15–64 years), the highest number of cases was reported during winter (44.3%). The disease was prevalent with ultraviolet radiation over 17MJ/m2 (67.8%; p=<0.001); relative humidity between 31.0% and 69.0% (95.8% of cases; p=<0.00); 12h of daily sunlight or more (40.6%; p=0.001); and temperatures between 20°C and 23°C (72.4%; p=<0.001). In the city of Taguatinga and surrounding area, pollution levels dropped to 15.2% between 2003 and 2012. Smoke levels decreased to 31.9%. In the Sobradinho region, particulate matter dropped to 13.1% and smoke to 19.3%, coinciding with the reduction of Tuberculosis incidence rates during the same period.
ConclusionThe results should guide surveillance actions for Tuberculosis control and elimination and indicate the need to expand observation time to new climate indicators and air quality.
Although Brazil is among the 22 countries with the highest TB burden (35.4/100,000 inhabitants), the Brazilian Midwest Region (MW) presents a low-tuberculosis burden scenario (24.1/100,000 inhabitants). The Federal District (FD) features 13 cases per 100,000 inhabitants and an annual decrease of 2.2%, indicating a trend toward pre-elimination of the disease.1
Social limitations,2 vitamin D deficiency,3–6 comorbidities,7 and limited access to health services8 are risk factors for developing TB. In addition, ecological studies conducted in countries where the incidence of TB was high relate the magnitude of TB to climatic summer factors – Spain9,10; Peru3; India10,11; Cape Town, South Africa12; United Kingdom, Wales and Scotland4,10,13; and South Africa, Kuwait, Ireland and Mongolia.10 In Hong Kong, TB reports were high in sputum-smear or culture positive patients in the summer.10,14 TB cases increased in spring in New York15 and in the rainy season in Cameroon.16 In Japan, the seasonality of TB varied according to clinical form and age, being higher in the spring among AFB+ ganglionar TB young patients (late spring to summer) and in AFB+ elderly in summer.17 In Cape Town, South Africa, TB affected more children in the spring,12 whereas in Spain was in the winter.18 In addition, studies revealed that the less ultraviolet light exposure, the more frequent is TB, as verified in Australia5,19 because of vitamin D deficiency.20 In contrast, in Peru the development of TB was higher in the summer due to the rainy period with low sunlight incidence.3
Other climatic factors such as temperature,21 precipitation, and humidity can influence the development of Mycobacterium tuberculosis.22 Air quality is affected by atmospheric pollution, where carbon monoxide induces bacillary reactivation23 and increases the incidence of tuberculosis.24 In addition, large seasonal amplitudes of TB often occur in upland regions with temperate mountain climate and low annual average temperature.21 Therefore, findings described in the literature confirm the relevance of conducting a study to better understand how climate and air quality can influence TB development in the Federal District. The objective of this study was to analyze the relationship between climatic factors and air quality with tuberculosis in the Federal District of Brazil (2003–2012). Through this analysis, we suggest improvements in the accuracy of the monitoring system and in the planning and allocation of resources to activities of the TB control program, taking into consideration the global climate change context.
MethodologyThe study was conducted in the Federal District (FD), the capital city of Brazil, located in the Midwest region. The FD has an area of 5,778,999km2 and is divided into 31 administrative regions25 (AR), with the health sector distributed into 15 Health Districts. The public service is responsible for 79.9% of all health actions26 and is the only entity to provide TB treatment.
The FD has a population of 2,957,954 inhabitants,25 96.6% of whom are living in urban areas.26 High-altitude tropical climate prevails in the region, with wet and rainy summers, dry and cold winters, and relative humidity of ≤20.0%. The average annual temperature is 21°C, with an average high of 35.8°C and an average low of 16°C. From 2003 to 2012, solar ultraviolet radiation in the FD showed a variation of 17–20MJ/m2.27 The region has little cloudiness and an average 75.0% days of sunshine during the year. During the period analyzed in our study, in general air quality in the FD was considered good.28
We analyzed 3927 TB cases (pulmonary, extrapulmonary, and pulmonary+extrapulmonary) of patients in the FD registered under the information system of health events (SINAN/TB), part of the Federal District Health Department (DF). We excluded non-residents and cases without address identification (0.4% excluded). Health Centers in the FD are 70.0% public and free-of-charge,1 and 67.3% of the Centers manage tuberculosis cases.1
We analyzed climate variables, air quality indicators, and demographic data from 2003 to 2012. Secondary data was obtained from the National Institute of Meteorology – INMET,29 Brazilian Agricultural Research Corporation – EMBRAPA,27 Brasilia Environmental Institute – IBRAN,28 Brazilian Institute of Geography and Statistics – IBGE,25 and the Federal District Planning Company – CODEPLAN.30
The variables included demographic data (gender, age, educational level, and race/skin color), climate (temperature), solar radiation levels, relative humidity, and TB incidence. Regarding air quality and pollution, four regions in the FD were analyzed: Taguatinga, Sobradinho, the North Wing, and the South Wing. Pollution monitoring sampling points were defined by IBRAN in order to prioritize areas with high traffic and population density.
A case was defined as direct smear and/or culture proven TB medium with histopathology confirmation or clinical and epidemiological findings suggestive of TB.1 We used the Köppen classification model31 for climate analysis and the national standards established by the CONAMA Resolution for air quality assessment (No. 3 of 28, 1990).28
Statistical analyses were performed using Pearson's chi-square test to check the dependence or independence of the variables32 with a 5% significance level. The study was approved by the Ethics Committee of the University of Brasilia, Opinion No. 1,098,421.
ResultsThe Federal District Health Department reported 4017 new cases of TB to SINAN-TB between 2003 and 2012. Out of the 4017 cases a total of 3927 were selected; 0.4% excluded: 52 had no address information and 38 for being residents of other states.
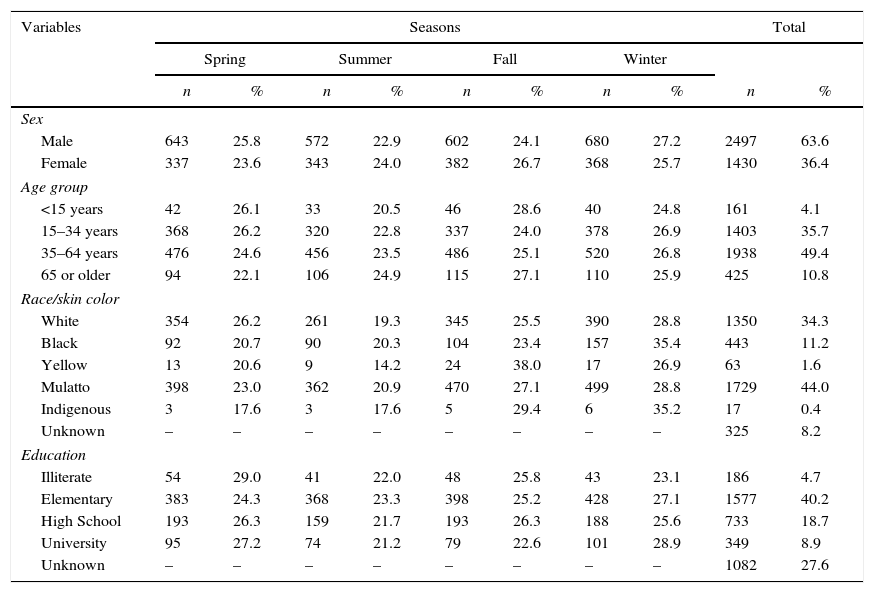
Among the demographic variables, there was a predominance of males (63.6% of cases). The most frequent age group was 15–64 years (53.7%), the most common level of education was primary school (40.2%), and the predominant race was mulatto (44.0%) (Table 1).
Demographic characteristics of new cases of tuberculosis per season from 2003 to 2012. Federal District, Brazil.
| Variables | Seasons | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Spring | Summer | Fall | Winter | |||||||
| n | % | n | % | n | % | n | % | n | % | |
| Sex | ||||||||||
| Male | 643 | 25.8 | 572 | 22.9 | 602 | 24.1 | 680 | 27.2 | 2497 | 63.6 |
| Female | 337 | 23.6 | 343 | 24.0 | 382 | 26.7 | 368 | 25.7 | 1430 | 36.4 |
| Age group | ||||||||||
| <15 years | 42 | 26.1 | 33 | 20.5 | 46 | 28.6 | 40 | 24.8 | 161 | 4.1 |
| 15–34 years | 368 | 26.2 | 320 | 22.8 | 337 | 24.0 | 378 | 26.9 | 1403 | 35.7 |
| 35–64 years | 476 | 24.6 | 456 | 23.5 | 486 | 25.1 | 520 | 26.8 | 1938 | 49.4 |
| 65 or older | 94 | 22.1 | 106 | 24.9 | 115 | 27.1 | 110 | 25.9 | 425 | 10.8 |
| Race/skin color | ||||||||||
| White | 354 | 26.2 | 261 | 19.3 | 345 | 25.5 | 390 | 28.8 | 1350 | 34.3 |
| Black | 92 | 20.7 | 90 | 20.3 | 104 | 23.4 | 157 | 35.4 | 443 | 11.2 |
| Yellow | 13 | 20.6 | 9 | 14.2 | 24 | 38.0 | 17 | 26.9 | 63 | 1.6 |
| Mulatto | 398 | 23.0 | 362 | 20.9 | 470 | 27.1 | 499 | 28.8 | 1729 | 44.0 |
| Indigenous | 3 | 17.6 | 3 | 17.6 | 5 | 29.4 | 6 | 35.2 | 17 | 0.4 |
| Unknown | – | – | – | – | – | – | – | – | 325 | 8.2 |
| Education | ||||||||||
| Illiterate | 54 | 29.0 | 41 | 22.0 | 48 | 25.8 | 43 | 23.1 | 186 | 4.7 |
| Elementary | 383 | 24.3 | 368 | 23.3 | 398 | 25.2 | 428 | 27.1 | 1577 | 40.2 |
| High School | 193 | 26.3 | 159 | 21.7 | 193 | 26.3 | 188 | 25.6 | 733 | 18.7 |
| University | 95 | 27.2 | 74 | 21.2 | 79 | 22.6 | 101 | 28.9 | 349 | 8.9 |
| Unknown | – | – | – | – | – | – | – | – | 1082 | 27.6 |
In the 10 years examined, the highest incidence of tuberculosis was in the winter (27.0%), followed by fall (25.0%), spring (24.7%), and summer (23.3%). Male patients showed higher incidence in winter (27.2%) and females in the fall (26.7%). There was a higher incidence in infants and children under 15 years in the fall (28.6%). Youth and adults (15–64 years of age) became ill more often in the winter (44.3%) while patients over 64 years in the fall (27.1%) (Table 1).
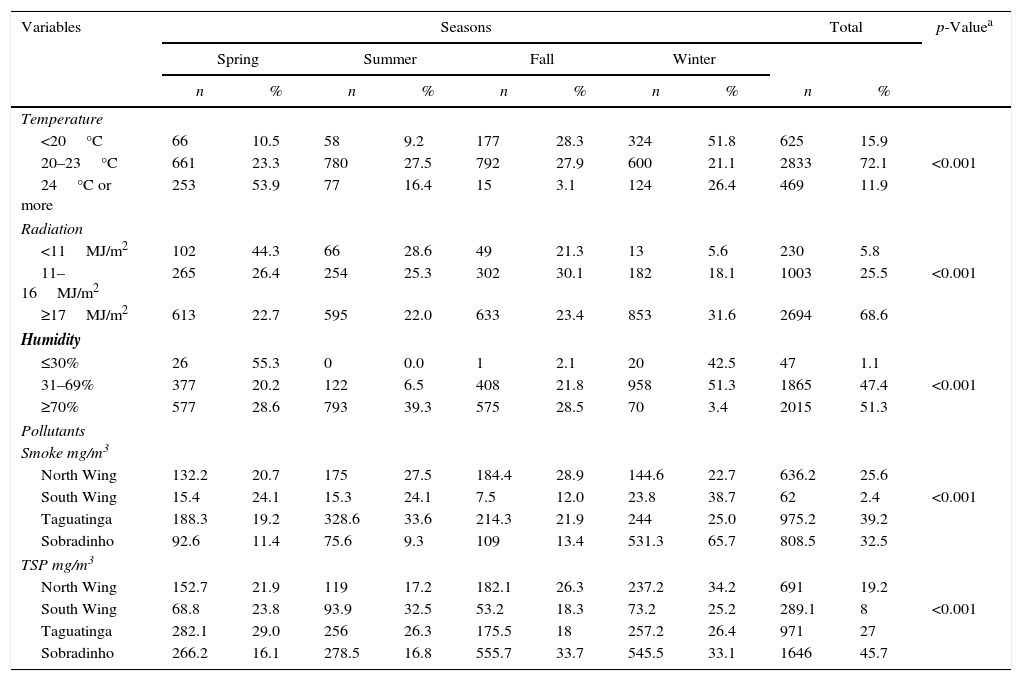
Considering climate variables in the FD, tuberculosis was more prevalent with ultraviolet radiation conditions (UVR) above 17MJ/m2 (67.8%; p=<0.001); relative humidity between 31.0% and 69.0% (95.8%; p=<0.001); precipitation values less than 1mm (71.7%; p=<0.001); daily sunlight exposure over 12h (40.6%; p=0.001); and temperature between 20°C and 23°C (72.4%; p=<0.001) (Table 2).
Climatic conditions per season during the study period of 2003–2012. Federal District, Brazil.
| Variables | Seasons | Total | p-Valuea | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Spring | Summer | Fall | Winter | ||||||||
| n | % | n | % | n | % | n | % | n | % | ||
| Temperature | |||||||||||
| <20°C | 66 | 10.5 | 58 | 9.2 | 177 | 28.3 | 324 | 51.8 | 625 | 15.9 | |
| 20–23°C | 661 | 23.3 | 780 | 27.5 | 792 | 27.9 | 600 | 21.1 | 2833 | 72.1 | <0.001 |
| 24°C or more | 253 | 53.9 | 77 | 16.4 | 15 | 3.1 | 124 | 26.4 | 469 | 11.9 | |
| Radiation | |||||||||||
| <11MJ/m2 | 102 | 44.3 | 66 | 28.6 | 49 | 21.3 | 13 | 5.6 | 230 | 5.8 | |
| 11–16MJ/m2 | 265 | 26.4 | 254 | 25.3 | 302 | 30.1 | 182 | 18.1 | 1003 | 25.5 | <0.001 |
| ≥17MJ/m2 | 613 | 22.7 | 595 | 22.0 | 633 | 23.4 | 853 | 31.6 | 2694 | 68.6 | |
| Humidity | |||||||||||
| ≤30% | 26 | 55.3 | 0 | 0.0 | 1 | 2.1 | 20 | 42.5 | 47 | 1.1 | |
| 31–69% | 377 | 20.2 | 122 | 6.5 | 408 | 21.8 | 958 | 51.3 | 1865 | 47.4 | <0.001 |
| ≥70% | 577 | 28.6 | 793 | 39.3 | 575 | 28.5 | 70 | 3.4 | 2015 | 51.3 | |
| Pollutants | |||||||||||
| Smoke mg/m3 | |||||||||||
| North Wing | 132.2 | 20.7 | 175 | 27.5 | 184.4 | 28.9 | 144.6 | 22.7 | 636.2 | 25.6 | |
| South Wing | 15.4 | 24.1 | 15.3 | 24.1 | 7.5 | 12.0 | 23.8 | 38.7 | 62 | 2.4 | <0.001 |
| Taguatinga | 188.3 | 19.2 | 328.6 | 33.6 | 214.3 | 21.9 | 244 | 25.0 | 975.2 | 39.2 | |
| Sobradinho | 92.6 | 11.4 | 75.6 | 9.3 | 109 | 13.4 | 531.3 | 65.7 | 808.5 | 32.5 | |
| TSP mg/m3 | |||||||||||
| North Wing | 152.7 | 21.9 | 119 | 17.2 | 182.1 | 26.3 | 237.2 | 34.2 | 691 | 19.2 | |
| South Wing | 68.8 | 23.8 | 93.9 | 32.5 | 53.2 | 18.3 | 73.2 | 25.2 | 289.1 | 8 | <0.001 |
| Taguatinga | 282.1 | 29.0 | 256 | 26.3 | 175.5 | 18 | 257.2 | 26.4 | 971 | 27 | |
| Sobradinho | 266.2 | 16.1 | 278.5 | 16.8 | 555.7 | 33.7 | 545.5 | 33.1 | 1646 | 45.7 | |
TSP, total supended particulate matter.
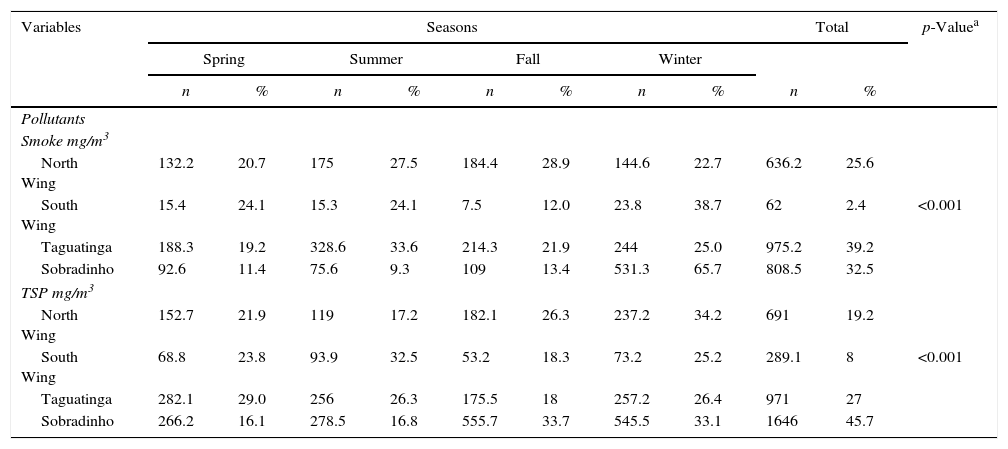
In relation to air quality, the two main pollutants were smoke (CO2, CO, SO2 and NO2) and total suspended particulate matter (TSP). Among the regions covered by the study (Taguatinga, Sobradinho, North Wing, and South Wing), pollution levels were the highest in Taguatinga and Sobradinho regions, both with hazardous levels of health concern – TSP≥375mg/m3 and smoke≥250mg/m3. We identified a greater amount of pollutants in the spring in Taguatinga region (38.5%), and in the winter in Sobradinho (50.0%) (Table 3). During the study period a drop of TSP and smoke rates in Taguatinga (−15.2% and −31.9%, respectively) was observed. Similarly, TSP and smoke levels reduced in Sobradinho (−13.1% and −9.3%, respectively). These decreases in pollution and smoke levels were associated with lower incidence rates (IR) of TB in the same period, with a reduction of 46% in Taguatinga and of 66.5% in Sobradinho. These findings suggest that the decrease of pollutants was associated with the reduction of TB cases in the region.
Characteristics of air quality per season during the study period of 2003–2012. Federal District, Brazil.
| Variables | Seasons | Total | p-Valuea | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Spring | Summer | Fall | Winter | ||||||||
| n | % | n | % | n | % | n | % | n | % | ||
| Pollutants | |||||||||||
| Smoke mg/m3 | |||||||||||
| North Wing | 132.2 | 20.7 | 175 | 27.5 | 184.4 | 28.9 | 144.6 | 22.7 | 636.2 | 25.6 | |
| South Wing | 15.4 | 24.1 | 15.3 | 24.1 | 7.5 | 12.0 | 23.8 | 38.7 | 62 | 2.4 | <0.001 |
| Taguatinga | 188.3 | 19.2 | 328.6 | 33.6 | 214.3 | 21.9 | 244 | 25.0 | 975.2 | 39.2 | |
| Sobradinho | 92.6 | 11.4 | 75.6 | 9.3 | 109 | 13.4 | 531.3 | 65.7 | 808.5 | 32.5 | |
| TSP mg/m3 | |||||||||||
| North Wing | 152.7 | 21.9 | 119 | 17.2 | 182.1 | 26.3 | 237.2 | 34.2 | 691 | 19.2 | |
| South Wing | 68.8 | 23.8 | 93.9 | 32.5 | 53.2 | 18.3 | 73.2 | 25.2 | 289.1 | 8 | <0.001 |
| Taguatinga | 282.1 | 29.0 | 256 | 26.3 | 175.5 | 18 | 257.2 | 26.4 | 971 | 27 | |
| Sobradinho | 266.2 | 16.1 | 278.5 | 16.8 | 555.7 | 33.7 | 545.5 | 33.1 | 1646 | 45.7 | |
TSP, total supended particulate matter.
Regarding the demographic profile, the highest incidence of TB in the FD occurred in young adult male patients, with elementary education, and of mulatto race. Likewise, 67.0% of the Brazilian TB patients were young adult males.1 In Cameroon, TB was significantly more prevalent in males (12.6%) compared to females (10.7%).16 Peru,3 China,23 Spain,18 and the United States21 showed similar results.
In the present study, the highest incidence of cases in children under 15 years of age in the FD occurred in the fall, a season characterized by mild temperatures and long periods without rain. The result is similar to that reported in the United States, where there was a peak of childhood TB from spring to late fall.21 The studies suggest a season interval for the transmission of TB from adults to children since, in general, childhood TB peaks in the next season following adult TB peak.12 Youth and adults (15–64 years of age) develop more TB in the winter (53.7%) and patients over 64 in the fall (27.1%). This is somewhat similar to what happened in China, where the incidence was 15.7% in children under 15 years of age and 34.0% in the age range of 15–64 years, predominantly during the winter and fall.33 In this study, children under 15 years of age developed less TB, despite their physiological disadvantages, such as decreased cardiac output, accelerated metabolism, developing immune system, and other forms of age-level developmental characteristics.34 Out of TB patients in China 50.3% were older than 64, where life expectancy is 75.7 years.33 Similar events were observed in Japan,4 United Kingdom14 and the United States,21 where life expectancies were 83, 81, and 78 years, respectively.
Several authors underscore an association of low education level and TB.2,35,36 One study in Spain indicated that 53.8% of the patients had not completed primary education.37 In China,38 patients with less than six years of schooling had a risk of relapse 3.4 times higher, and a risk of defaulting 4.3 times greater in newly treated TB cases. In the FD, TB was more prevalent in the least educated group (<8 years of education; 44.9%), reinforcing the association of lower level of education and the disease, highlighting that 27.6% of the responses were ignored. Considering race in Brazil,25 mulatto was the most frequent race seen in TB patients of the FD.
Seasonality analysis revealed that TB was more prevalent in the winter in the FD, similarly that observed in other regions of the country.39 In the United Kingdom,4 reduced sun exposure in the winter could decrease the host's defense to the tubercle bacillus because of vitamin D deficiency. Temperatures in the winter in the Midwest region of Brazil vary from 12°C to 27°C. It is worth noting that in Brazil the four seasons are not markedly different as they are in other regions in the world. A seasonal pattern of TB with a predominant peak during the fall was observed in the FD. A similar pattern was verified in Peru,3 where the diagnosis of tuberculosis increases in late summer and early fall because of the rainy characteristics of those seasons. High humidity and absence of direct sunlight due to cloudiness are similar characteristics of the Brazilian autumn.
A high level of UV radiation was observed in the FD, averaging 17MJ/m2 with 12h/day of incident sunlight (40.6%; p=0.001). This probably occurs due to geographical aspects of the FD – plateau relief with smooth topography – that facilitate the penetration of sunlight. This fact should justify the lower TB incidence rates in the region compared to other areas of the country.1 TB incidence peaks in the winter in Peru coincide with the low sunlight periods due to vitamin D deficiency.3,5,6,40,41 In England,13 low levels of vitamin D in post-winter might result in an impairment of cellular immunity leading to reactivation of mycobacterial infection after a period of latency.
In the FD, 95.8% of TB cases occurred with relative humidity between 31.0% and 69.0% and precipitation values less than 1mm (71.7%; p=<0.001). It is noteworthy that European humidity is high (70.0% average) due to high rainfall caused by winds that bring moisture from the ocean to the continent almost all year round.4 In Cameroon, more TB cases were recorded in the rainy season, with a significant difference as compared to other seasons.16 Similarly, a greater incidence of TB in Mongolia occurs in wet seasons.22
Possible explanations for seasonal variations in the incidence of TB include decreased vitamin D levels in winter,6,40–42 which leads to depression of immune response and consequent reactivation of TB. Another risk factor for TB in winter is household crowding,15 which may be found in the poorest areas in the FD. It is noteworthy that direct sunlight is present during 75.0% of the days in the FD.29 High solar radiation may explain the lower incidence of tuberculosis in the region compared to other areas of the country.
More cases of TB were reported in the FD when the temperature was between 20°C and 23°C (72.4% of cases), in line with several studies carried out in different places, including: New York (20–25°C)15; Spain (16–24°C)9; Cape Town, South Africa (13–23°C)41; UK (11.7°C and 21.1°C)4; and Peru.3 However, TB was also diagnosed at higher temperatures: 39°C in Cameroon16; 21–39°C in Northern India11; and 20–38°C in Kuwait.43 Lower temperatures have been identified in Japan (5°C)44 and Mongolia (−5°C to 9°C).22 In general, TB incidences were higher in milder temperatures, similar to conditions reported in the FD.
In relation to air quality, two main pollutants were identified in the FD: smoke (CO2, CO, SO2, and NO2) and total suspended particulate matter (TSP), especially in Taguatinga and Sobradinho. In Sobradinho TB notifications were higher in winter; in Taguatinga in spring. Although the FD has 15 health districts, only four points could be sampled for air quality analysis: the South Wing, the North Wing, Taguatinga, and Sobradinho. It is important to mention that air quality improved in the FD as a result of a Resolution (CONAMA No. 242/1998) that regulates modes of transportation and actions issued by the National Council for the Environment to control air quality (provided in CONAMA Resolution No. 436/2011 prohibiting the emission of pollutants in the federal capital by industries). Despite the limitation of air quality monitoring equipment in the FD (only four), the data seem to indicate that air pollution is directly related to TB incidence. Studies in the US and Russia also suggest a link between concentrations of smoke and total suspended particulate matter and tuberculosis, especially considering the amounts of NO2 and CO2.24,45 Air pollution generated by traffic in Taiwan – caused by sulfur dioxide, ozone, and carbon monoxide – was associated with culture-confirmed TB.46 Likewise, a study in South Korea revealed that long-term exposure to ambient SO2 increased the risk of TB by 7.0% in males.47
In this study, Taguatinga and Sobradinho showed levels of great health concern; i.e., TSP pollution ≥375mg/m3 and smoke ≥250mg/m3. A higher amount of pollutants was reported in Taguatinga (38.5%) in spring and in Sobradinho in winter (50.0%). In South Korea, the exposure to high concentrations of suspended particles in the atmosphere increased at 1.27 times the incidence of TB48 and the exposure to fine particulate matter (PM 2.5) was associated with increased risk of the disease.49 In Taiwan, exposure suspended particles increased the rate of TB by 4.0% and interfered with smear results. The chronic exposure to ≥50μg/m3 PM 10 may prolong the sputum culture conversion of TB patients with sputum-positive culture.38
Among the limitations of the study is the use of secondary bases of SINAN-TB since the information can be inconsistent due to incomplete records and/or missing data leading to methodological bias. Other limitations were the lack of more effective air pollution assessments in different regions of the FD and the analysis of air quality restricted to smoke and to certain particulate matter.
In summary, the results suggest that the incidence of TB appears to have been affected not only by climatic factors – seasons and climatic variables, solar radiation, temperature, humidity, and air quality – but also by social and demographic factors – age, gender, race, and education. However, we underscore the need for further studies so that the role of other environmental variables can be clarified. Air-quality observation time should also be increased so that we can better understand the relationship of TB with the weather.
Conflicts of interestThe authors declare no conflicts of interest.