The aims of this study were to determine the incidence of external genital lesions (EGLs), specifically histologically confirmed condyloma (genital warts) and Penile Intraepithelial Neoplasia (PeIN), and genital HPV infection progression to EGLs among healthy men aged 18–73 residing in Brazil. Subjects included 1118 men enrolled in the HPV Infection in Men (HIM) study between July 2005 and June 2009. At each visit, EGLs were biopsied and subjected to pathological evaluation. HPV status in genital swabs and biopsies was determined by Linear Array and INNO-LiPA, respectively. Age-specific EGLs incidence and the proportion and median time to EGL development were estimated. Kaplan–Meier cumulative incidence rates at 6, 12, and 24 months were determined. During follow-up, 73 men developed an incident EGL. Men could develop multiple EGLs and there were 36 men with condyloma, 27 men with lesions suggestive of condyloma, six men with PeIN, and 20 men with non-HPV lesions. HPV-positive men who developed EGLs were younger (p=0.002) than men that did not develop lesions. Among the 815 men with HPV infection, 4% progressed to EGL with the same HPV detected in the swab. During follow up, 15.7% of genital HPV-6 and HPV-11 infections progressed to condyloma (median progression time of nine months for HPV-6 versus 6.8 months for HPV-11). Approximately 1% of HPV-16 infections progressed to PeIN with a median progression time of 25 months. HPV types covered by the 4-valent HPV vaccine were detected in 82.3% and 83.3% of condyloma and PeIN, respectively. The high burden of HPV and high frequency of progression to disease underscores the need to offer HPV prophylactic vaccination to men to reduce the overall burden of infection and diseases caused by HPV.
Human papillomavirus (HPV) is the most common sexually transmitted infection worldwide with sexual activity constituting the major risk factor for infection.1 Most infections are asymptomatic and clear spontaneously within two years. However, persistence of high-risk HPV is a strong predictor of cervical and other HPV-related cancers development. To date, more than 200 HPV genotypes have been identified, 40 of which are routinely detected in anogenital specimens of both females and males.2 Genital HPV infection is very common in men3 and infections may progress to external genital lesions (EGLs), mostly condylomata acuminata (genital warts or condyloma) and penile intraepithelial neoplasia (PeIN) that are thought to precede penile cancer.4
Condyloma is a common clinical outcome of HPV infection in males, mainly among men aged 25–29 years.5 Nononcogenic HPV-6 and HPV-11 are the etiologic agents of over 90% of condylomas.6 Although about one third of these warts spontaneously regress, recurrence is common, therefore, treatment results in high medical costs.7 The incidence of condyloma among Brazilian men is poorly known. While there are some reports on genital HPV infection in man,8–10 the incidence of HPV infection and condyloma in Brazilian men are limited to few publications.11–13
Penile cancer occurs more frequently in men aged 50–70 years, with a global estimate of 22,000 cases per year.1 Though this cancer is rare, incidence rates are higher in less developed countries representing up to 10% of tumors in man in some parts of Africa, Asia, and South America.14 In Brazil, this neoplasia accounts for about 2% of all cancers in man, where both the North and Northeast regions are mostly affected.15 Both benign and malignant HPV-related tumors in men are potentially preventable with the 4-valent HPV vaccine.16,17
The purpose of this study was to estimate EGL incidence and assess the rate of progression from HPV infection to histopathologically confirmed EGLs in otherwise healthy men from Brazil participating in an international HPV natural history study, the HPV Infection in Men (HIM) Study.
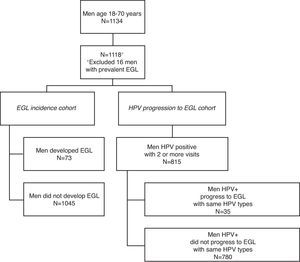
Materials and methodsStudy designThe HIM Study includes over 4,000 men aged 18–70 years enrolled between 2005 and 2009 in São Paulo (Brazil), Cuernavaca (Mexico), and Tampa, Florida (USA). Study methods and design have been described in detail elsewhere.3 In brief, every six months participants underwent interview, a physical exam, and laboratory analysis of anogenital specimens for HPV detection. In 2009 a biopsy and pathology protocol was implemented allowing for pathological confirmation and HPV genotyping of lesions. Men who had two or more study visits after the implementation of this protocol were included in the present study.
In São Paulo, the largest city of Brazil, a total of 1443 men were recruited at technical and higher education institutions, in communities, companies and military corporations, through leaflets posters, letters and e-mails addressed to participants of other studies. Men were also invited through some radio and internet ads and during counseling sessions on a public STD (sexually transmitted disease) clinic. Men in treatment for STDs or condyloma, and HIV-infected individuals were excluded from participating in the study. Retention rates in Brazil were the highest among all three sites participating at the HIM study: 80% of all enrolled men completed 10 visits of follow-up in approximately five years, according to the study protocol (six-month interval visits). The study was approved by the ethical review boards of the Institutional Review Boards at the University of South Florida (Tampa, FL, USA), the Ludwig Institute for Cancer Research (São Paulo Branch, Brazil), Centro de Referência e Treinamento em DST/Aids (São Paulo, Brazil), and the Instituto Nacional de Salud Publica (Cuernavaca, Mexico). Informed consent was obtained from all participants.
Clinical specimens, HPV detection and genotypingAt each visit, three different pre-wetted Dacron swabs were used to sample the external genitalia (coronal sulcus, glans penis, penile shaft) and scrotum of participants, and were later combined to form a single sample.3 Specimens underwent DNA extraction using the Qiagen Media kit (Qiagen, Venlo, The Netherlands), and HPV detection and typing was conducted using the Linear Array® HPV Genotyping Test (Roche Molecular Diagnosis, Pleasanton, USA), capable of identifying 37 HPV types, classified as high-risk (HR-HPV: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68) or low-risk (LR-HPV: 6, 11, 26, 40, 42, 53, 54, 55, 61, 62, 64, 66, 67, 69, 70, 71, 72, 73, 81, 82, IS39, 83, 84, 89).
Furthermore, at each clinic visit participants underwent clinical examination under 3× light magnification by a trained clinician in order to detect EGLs. Whenever a lesion was observed, a tissue sample was obtained by shave excision, fixed and sent for histopathological evaluation. All EGLs that appeared to be HPV-related or had an unknown etiology based on visual inspection were sent for HPV testing. EGLs were categorized as condyloma, suggestive of condyloma (very early condylomas that have some but have not yet developed all of the pathological features of condyloma), PeIN, or not HPV-related (such as skin tags, seborrheic keratosis, chronic balanitis, Molluscum contagiosum), according to criteria previously published.13 PeIN lesions were further categorized in PeIN I (low-grade squamous intraepithelial lesion [SIL]), PeIN II (high-grade SIL), PeIN II/III (high-grade SIL), and PeIN III (high-grade SIL). DNAs from formalin-fixed, paraffin-embedded (FFPE) tissues were extracted using the QIAamp DNA FFPE Tissue Kit (Qiagen, Venlo, The Netherlands), and HPV detection and genotyping was conducted using the INNO-LiPA HPV Genotyping Extra assay (Fujirebio, Göteborg, Sweden) which detects 28 viral types (HR-HPV: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68; or LR-HPV: 6, 11, 26, 40, 43, 44, 53, 54, 66, 69, 70, 71, 73, 74, 82). DNAs from swabs and tissues were considered adequate and included in this study if tested positive for β-globin or any HPV genotype.
Statistical analysisEGL incidenceAll men with prevalent lesions were excluded from the biopsy cohort. Age-specific analyses were conducted among men who developed an incident EGL, and age groups were stratified as 18–30, 31–44, and 45–73 years. For EGL incidence analyses, only the first detected EGL was considered. Time to incident EGL was calculated from biopsy cohort baseline date to the date of first EGL detection. Person-time incidence and 95% confidence intervals (CIs) were calculated based on the number of events modeled as a Poisson variable for the total number of person-months. Kaplan–Meier curves for EGL incidence were generated, and the incidence of EGL over time across the three age groups was compared using the log-rank test.
To evaluate HPV distribution in EGLs, all prevalent and incident lesions were included. In addition to specific HPV types, infections positive for ≥1 type were included in the any HPV group, those positive for ≥1 high-risk HPV type were included in the high-risk HPV group, and those positive for ≥1 low-risk types were included in the low-risk HPV group. Independent analyses were conducted for high-risk and low-risk infections. Additionally, EGLs that were positive for ≥1 high-risk type and ≥1 low-risk type were included in both HR/LR HPV group.
HPV progression to EGLSociodemographic and sexual behavior characteristics were described for HPV positive men who did or did not develop an EGL during follow-up, and comparisons were performed using the Monte Carlo estimation of exact Pearson chi-square tests.
Men with an incident or prevalent genital HPV infection and without a prevalent condyloma or PeIN lesion at the biopsy protocol baseline visit were included in the analyses designed to assess the rate of progression and the proportion of infections progressing to disease. HPV infection was reported by genotype or grouped (any, HR-HPV, LR-HPV, and vaccine (HPVs 6/11/16/18)). The classification of any HPV type was defined as a positive test result for at least one of 25 (HPV types 43/44/74 are not detected by the Linear Array assay) HPV genotypes detected by INNO-LiPA. HPV infections with single or multiple HR-HPV types were classified as HR and those with at least one LR-HPV type were classified as LR.
Time-to-event approach was applied to assess the time from type-specific genital HPV positivity to EGL incidence harboring the same HPV type within the lesion. The analytical unit for this study is infection. HPV genital infections that did not progress to EGL were censored at the last visit. The 6-, 12-, and 24-month cumulative incidence of EGLs and median time to EGL development for individual genital HPV types were estimated using the Kaplan–Meier method. For grouped genital HPVs, we adjusted for within-subject correlation using the clustered Kaplan–Meier method as men could have been infected with multiple HPV types within a defined group. The overall EGL incidence rate during the study period was also calculated. Multiple HPV types could be detected in a single EGL, and a man could develop multiple EGLs.
ResultsEGL incidenceOverall 1134 Brazil HIM Study participants aged 18–73 years were included in the biopsy cohort. Sixteen men with a prevalent EGL were excluded from the EGL incidence analysis (Fig. 1). Among the remaining 1118 men, 73 developed at least one incident EGL during follow up. Men primarily developed condyloma (n=36), suggestive of condyloma (n=27), PeIN (n=6), or non-HPV related EGL (n=20).
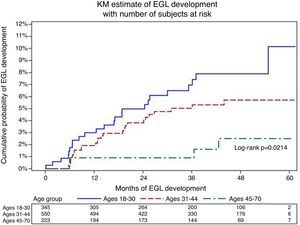
Table 1 presents EGL incidence stratified by age. There was a decline in the 12-month cumulative incidence for any EGL by age (3.4%, 1.9%, and 0.9% for ages 18–30, 31–44, and 45–73 years, respectively; p=0.02; Fig. 2). When stratified by lesion type, the 12-month cumulative condyloma incidence was higher among men <30 years of age (1.8% among men aged 18–30, p=0.06) compared to older men. In contrast, among men who developed PeIN, the 12-month cumulative incidence remained constant across categories of age (0.6%, 0.0%, and 0.0% for ages 18–30, 31–44, and 45–73 years, respectively; p=0.38 across the entire follow-up period).
Age-specific incidence of pathologically confirmed condyloma among men from Brazil.
| Pathological diagnosis | |||||
|---|---|---|---|---|---|
| Anya | Condyloma | Suggestiveb | PeINc | Otherd | |
| All ages (n=1118)e | |||||
| Men with incident EGL, no. | 58 | 36 | 27 | 6 | 20 |
| Person-months | 41,109 | 41,760 | 41,743 | 42,407 | 42,079 |
| Incidence ratef (95% CI) | 1.69 (1.31–2.19) | 1.03 (0.75–1.43) | 0.78 (0.53–1.13) | 0.17 (0.08–0.38) | 0.57 (0.37–0.88) |
| 12-month incidence | 2.2 (1.4–3.3) | 1.1 (0.6–2) | 1 (0.6–1.9) | 0.2 (0–0.7) | 0.7 (0.3–1.4) |
| 18–30 y (n=345) | |||||
| Men with incident EGL, no. | 25 | 17 | 11 | 3 | 12 |
| Person-months | 12,504 | 12,794 | 12,822 | 13,092 | 12,895 |
| Incidence ratef (95% CI) | 2.4 (1.62–3.55) | 1.59 (0.99–2.56) | 1.03 (0.57–1.86) | 0.28 (0.09–0.85) | 1.12 (0.63–1.97) |
| 12-month incidence | 3.4 (1.9–6.1) | 1.8 (0.8–4.1) | 1.2 (0.5–3.2) | 0.6 (0.2–2.4) | 1.2 (0.5–3.2) |
| 31–44 y (n=550) | |||||
| Men with incident EGL, no. | 29 | 16 | 15 | 3 | 6 |
| Person-months | 20,322 | 20,646 | 20,611 | 20,968 | 20,851 |
| Incidence rate (95% CI) | 1.71 (1.19–2.46) | 0.93 (0.57–1.52) | 0.87 (0.53–1.45) | 0.17 (0.06–0.53) | 0.35 (0.16–0.77) |
| 12-month incidence | 1.9 (1–3.5) | 0.9 (0.4–2.3) | 1.1 (0.5–2.5) | 0.0 (0.0–0.0) | 0.6 (0.2–1.8) |
| 45–73 y (n=223) | |||||
| Men with incident EGL, no. | 4 | 3 | 1 | 0 | 2 |
| Person-months | 8283 | 8320 | 8310 | 8347 | 8333 |
| Incidence rate (95% CI) | 0.58 (0.22–1.54) | 0.43 (0.14–1.34) | 0.14 (0.02–1.03) | 0.0 (0.0–0.0) | 0.29(0.07–1.15) |
| 12-month incidence | 0.9 (0.2–3.8) | 0.5 (0.1–3.3) | 0.5 (0.1–3.3) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| p-Valueg | 0.02 | 0.06 | 0.10 | 0.38 | 0.02 |
Men with ≥1 incident, pathologically confirmed HPV-related EGL throughout the study period. For men with >1 EGL, incidence rates for the Any EGL category are determined; for the first detected lesion; thus, men may contribute fewer person-months in this category than for specific pathological diagnoses.
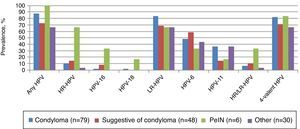
Any HPV DNA was detected in 87.3% of condyloma, and 83.5% were positive for low-risk viral types. HPV-6 and HPV-11 were detected in 48.1% and 36.7% of condylomas, respectively (Fig. 3). Less frequently detected low-risk types include HPVs 26, 40, 44, 54, 66, 74. Among 48 EGLs categorized as suggestive of condyloma, 72.9% were positive for any HPV, and the most common type detected was HPV-6 (58.3%). Among the six prevalent and incident PeIN lesions, 100% were HPV positive and 66.7% were positive for ≥1 HR-HPV genotype. HPV-16 and HPV-18 were detected in two (33.3%) and one (16.7%) of the PeIN lesions, respectively. Additionally two other HR-HPVs were also detected in PeIN lesions: HPV-39 was found in two samples whereas HPV-68 was detected in one sample (data not shown). LR-HPV types were mostly found in PeIN lesions as co-infections with a HR-HPV; however HPV-6 was detected as a single infection in one PeIN I and one PeIN III lesions. PeIN lesions occurred at the coronal sulcus (two cases), the glans penis (two cases), the meatus and the shaft left dorsal (1 case each) (Table 1). At least one of the HPV types in the 4-valent HPV vaccine was detected in 82.3%, 70.8%, and 83.3% of condyloma, suggestive of condyloma, and PeIN lesions, respectively.
HPV progression to EGLAmong HPV-positive men, EGL incidence was associated only with younger age (p=0.002) (Table 2). No other sociodemographic or sexual behavior variable was associated with EGL incidence.
Comparison of sociodemographic and sexual behavior characteristics between human papillomavirus-positive men who did and did not develop an external genital lesion (EGL) during follow-up in the HIM study in Brazil.
| No EGL incidence | Any EGL incidence | pa | |
|---|---|---|---|
| n (%) | n (%) | ||
| Age | 0.002 | ||
| 18–30 | 240 (30.8%) | 19 (54.3%) | |
| 31–44 | 392 (50.3%) | 15 (42.9%) | |
| 45–74 | 148 (19%) | 1 (2.9%) | |
| Race | 0.90 | ||
| White | 469 (60.1%) | 23 (65.7%) | |
| Black | 230 (29.5%) | 10 (28.6%) | |
| Asian/PI | 14 (1.8%) | 0 (0%) | |
| Other | 58 (7.4%) | 2 (5.7%) | |
| Refused | 9 (1.2%) | 0 (0%) | |
| Ethnicity | 0.68 | ||
| Hispanic | 193 (24.7%) | 8 (22.9%) | |
| Non-Hispanic | 570 (73.1%) | 27 (77.1%) | |
| Refused | 17 (2.2%) | 0 (0%) | |
| Years of education | 0.82 | ||
| Completed 12 years or less | 379 (48.6%) | 15 (42.9%) | |
| 13–15 years | 150 (19.2%) | 8 (22.9%) | |
| Completed at least 16 years | 247 (31.7%) | 12 (34.3%) | |
| Refused | 4 (0.5%) | 0 (0%) | |
| Missing | 0 (0%) | 0 (0%) | |
| Marital status | 0.23 | ||
| Single | 302 (38.7%) | 19 (54.3%) | |
| Married/cohabiting | 381 (48.8%) | 12 (34.3%) | |
| Divorced/separated/widowed | 95 (12.2%) | 4 (11.4%) | |
| Refused | 2 (0.3%) | 0 (0%) | |
| Missing | |||
| Circumcised | 0.82 | ||
| Not circumcised | 645 (82.7%) | 28 (80%) | |
| Circumcised | 135 (17.3%) | 7 (20%) | |
| Vaginal condom use | 0.21 | ||
| No sex | 105 (13.5%) | 1 (2.9%) | |
| Always | 140 (17.9%) | 6 (17.1%) | |
| Sometimes | 297 (38.1%) | 18 (51.4%) | |
| Never | 207 (26.5%) | 9 (25.7%) | |
| Missing | 31 (4%) | 1 (2.9%) | |
| Anal condom use | 0.12 | ||
| No Sex | 397 (50.9%) | 13 (37.1%) | |
| Always | 130 (16.7%) | 10 (28.6%) | |
| Sometimes | 121 (15.5%) | 8 (22.9%) | |
| Never | 126 (16.2%) | 4 (11.4%) | |
| Missing | 6 (0.8%) | 0 (0%) | |
| Smoking status | 0.48 | ||
| Current | 161 (20.6%) | 10 (28.6%) | |
| Former | 239 (30.6%) | 9 (25.7%) | |
| Never | 380 (48.7%) | 16 (45.7%) | |
| Missing | 0 (0%) | 0 (0%) | |
| Alcohol per month | 1.00 | ||
| 0 | 155 (19.9%) | 7 (20%) | |
| 1–30 | 314 (40.3%) | 14 (40%) | |
| >30 | 285 (36.5%) | 13 (37.1%) | |
| Missing | 26 (3.3%) | 1 (2.9%) | |
| Sexual orientationa | 0.61 | ||
| MSM | 41 (5.3%) | 1 (2.9%) | |
| MSMW | 210 (26.9%) | 10 (28.6%) | |
| MSW | 501 (64.2%) | 24 (68.6%) | |
| Missing | 28 (3.6%) | 0 (0%) | |
| Total number of female partners | 0.38 | ||
| 0–1 | 74 (9.5%) | 4 (11.4%) | |
| 2–9 | 167 (21.4%) | 4 (11.4%) | |
| 10–49 | 380 (48.7%) | 21 (60%) | |
| 50+ | 131 (16.8%) | 6 (17.1%) | |
| Refused | 28 (3.6%) | 0 (0%) | |
| Total number of male partners | 1.00 | ||
| 0 | 514 (65.9%) | 24 (68.6%) | |
| 1–9 | 164 (21%) | 7 (20%) | |
| 10+ | 87 (11.2%) | 4 (11.4%) | |
| Missing | 15 (1.9%) | 0 (0%) | |
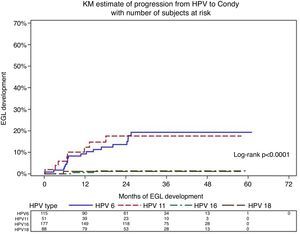
The HPV-6 positive condyloma incidence rate among men with a previously detected HPV-6 infection was 5.9 per 1000 person-months (Table 3). Among HPV-6 infections, 7.34% progressed to HPV-6-positive condyloma during the first six months of follow-up. For HPV-11, the incidence rate for HPV-11-positive condyloma was higher than HPV-6 at 6.7 per 1000 person-months, and the 6-month cumulative incidence was also higher at 10.07% (Fig. 4). The HPV-16 positive PeIN incidence rate was 0.4 per 1000 person-months among men with a prior HPV-16 infection. By 24 months of follow-up 0.79% of men with a genital HPV-16 infection progressed to an HPV-16-positive PeIN.
| HPV typed | Incidence rateg (95% CI) | Cumulative incidence (%) | ||
|---|---|---|---|---|
| 6m (95% CI) | 12m (95% CI) | 24m (95% CI) | ||
| Condylomaa,e | ||||
| Any | 0.5 (0.4–0.7) | 0.70 (0.42–1.15) | 1.05 (0.69–1.59) | 1.74 (1.23–2.44) |
| High risk | 0.1 (0.1–0.3) | 0.08 (0.01–0.57) | 0.17 (0.04–0.67) | 0.39 (0.14–1.05) |
| 16 | 0.4 (0.1–1.5) | 0.0 (0.0–0.0) | 0.64 (0.09–4.43) | 1.36 (0.34–5.35) |
| 18 | 0.4 (0.1–2.7) | 1.14 (0.16–7.79) | 1.14 (0.16–7.79) | 1.14 (0.16–7.79) |
| 45 | 0.4 (0.1–3.1) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 2.13 (0.30–14.16) |
| 52 | 0.2 (0.0–1.3) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| Low risk | 1.0 (0.7–1.5) | 1.48(0.88–2.49) | 2.17 (1.41–3.35) | 3.44 (2.39–4.93) |
| 6 | 5.9 (3.7–9.4) | 7.34 (3.73–14.15) | 10.27 (5.81–17.79) | 17.83 (11.35–27.39) |
| 11 | 6.7 (3.3–13.3) | 10.07 (4.31–22.53) | 12.31 (5.72–25.43) | 17.59 (9.12–32.4) |
| 40 | 0.6 (0.1–4.0) | 1.69 (0.24–11.43) | 1.69 (0.24–11.43) | 1.69 (0.24–11.43) |
| 53 | 0.2 (0–1.3.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.77 (0.11–5.33) |
| 66 | 0.4 (0.1–1.5) | 0.0 (0.0–0.0) | 1.3 (0.33–5.09) | 1.3 (0.33–5.09) |
| Vaccineh | 2.4 (1.6–3.4) | 3.37 (2.01–5.63) | 4.67 (3–7.24) | 7.61 (5.29–10.90) |
| Penile intraepithelial neoplasiaa,f | ||||
| Any | 0.1 (0.0–0.2) | 0.05 (0.01–0.34) | 0.05 (0.01–0.34) | 0.11(0.03–0.44) |
| High risk | 0.1 (0.0–0.2) | 0 (0–0) | 0 (0–0) | 0.11 (0.02–0.78) |
| 16 | 0.4 (0.1–1.5) | 0 (0–0) | 0 (0–0) | 0.79 (0.11–5.5) |
| Low risk | 0.1 (0.0–0.3) | 0.11 (0.02–0.77) | 0.11 (0.02–0.77) | 0.11 (0.02–0.77) |
| 6 | 0.3 (0.0–2.0) | 0.92 (0.13–6.33) | 0.92 (0.13–6.33) | 0.92 (0.13–6.33) |
| 73 | 0.4 (0.1–3.0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Vaccineh | 0.2 (0.1–0.7) | 0.25 (0.03–1.74) | 0.25 (0.03–1.74) | 0.57 (0.14–2.27) |
CI, confidence interval.
HPV types 33/35/39/51/56/58/59/68/26/54/69/70/71/73/82 did not progress to a condyloma lesion; therefore, incidence rates and cumulative incidence could not be calculated.
The HIM study is the first international comparison of the distribution of 37 HPV genotypes in the genitals of men enrolled in three different countries. Among these individuals, HPV prevalence at the genitals was higher in Brazil (72.3%), than in the USA (61.3%) and Mexico (61.9%).3 Although HPV infection is a common finding in men, when considering all HIM participants, only 5% of these incident viral infections progressed to and EGL during follow-up.13 We herein present data comparing EGL incidence and progression of HPV infection to EGL among men enrolled in Brazil. During follow-up 73 men from Brazil developed an incident EGL including 36 condyloma, six PeIN, and 20 non-HPV related EGLs (e.g. skin tags, benign keratosis). HPV-positive men who developed EGLs were younger (p=0.002) than men that did not develop lesions. HPV infection was detected in 815 men; 4% of infections progressed to an EGL. During the follow up, 15.7% genital HPV-6 and HPV-11 infections progressed to condyloma (median progression time of nine months for HPV-6 versus 6.8 months for HPV-11). Approximately 1% of HPV-16 infections progressed to PeIN with a median progression time of 25 months. HPV types covered by the 4-valent HPV vaccine were detected in 82.3%, 70.8%, and 83.3% of condyloma, suggestive of condyloma, and PeIN, respectively.
We previously observed that for all men enrolled in the HIM study, factors strongly associated with condyloma development included age, high lifetime number of female or male sexual partners.5 In the present analysis restricted to Brazilian men, we observed that condyloma incidence significantly differed only by age (p=0.02): younger men (<30) developed more genital warts either when considering the whole population18 or solely HPV-positive individuals. Nevertheless, it should not be ignored that individuals aged 45–70 years continued to develop condyloma during follow-up which highlights that men remain susceptible to developing HPV-related EGLs throughout their lives. In the current study HPV DNA was detected in 87.3% of condyloma biopsies, in line with previous results of 91% HPV prevalence in condyloma samples of women enrolled in the placebo arm of a vaccine trial.19 HPV-6 and HPV-11 were the most prevalent viral types detected in condylomas in agreement with previous reports,20–23 although HPV-6 detection surpassed that of HPV-11 (48.1% versus 36.7%) in these samples.
About 16% of HPV-6 and HPV-11 infections progressed to a condyloma with the same viral type during follow-up; however median time to progression was higher among HPV-6 positive men (nine months for HPV-6 versus 6.8 months for HPV-11). Our results are in line with previous estimates of two weeks to eight months as the time required from initial HPV-6 or HPV-11 infections to progression to condyloma.7
Although PeIN was a rare event in this population, we were able to detect and genotype six prevalent and incident PeIN lesions. Histopathologically, three cases were classified as PeIN III, one case as PeIN II, and two cases as PeIN I. All PeIN lesions were HPV-positive; HPV-16 was detected in two cases, in agreement with previous studies reporting 88–100% of samples positive for HPV DNA, and about 40% of PeIN positive for HPV-16.24 It is interesting to notice that genital swabs collected prior to HPV-16 positive PeIN harbored multiple HPV infections. Among Brazilian men enrolled in the HIM study, 1.1% of HPV-16 infections progressed to HPV-16-positive PeIN in a median time of 25.6 months. Among LR-HPV genital infections, one HPV-6 and one HPV-73 infections progressed to PeIN. However, whereas the HPV-6 infection progressed to a PeIN within 6.7 months, the HPV-73 infection progression occurred after 30.5 months. Similar results are observed when pooling data across the three participating countries of the HIM study, namely Brazil, Mexico, and USA: 1.6% of HPV-16 infections progressed to PeIN in a median time of 19 months.13 In addition, in one of the PeIN cases, we detected exclusively HPV-6 and time from infection to progression was 6.7 months. The presence of low-risk HPVs in high-grade precancerous anogenital lesions has been also observed by others.25–27 Nevertheless, the limited number of PeIN detected limits further considerations.
Considering the HIM study cohort as a whole, during all six years of follow-up, 10 incident PeIN lesions were detected, from the USA (two) and from Mexico and Brazil (four each).13 Regional differences concerning the rates of HPV-related tumors in these countries are reported. In Brazil the incidence rate of penile cancer is 0.7–2.3/100,000 inhabitants, one of world's highest incidence rates for this neoplasia.28 The city of Recife in Pernambuco, Northeast region of the country has an incidence rate of penile cancer of 4.6/100,000 inhabitants. Unfortunately, there is still very incomplete data available in regards to HPV prevalence in penile cancer and its precursor lesions in Brazil. In one study, HR-HPV was detected in two cases of condyloma and two cases of PeIN (one PeIN I and one PeIN III), whereas just one case of PeIN I was HPV negative by Hybrid Capture.29
As with any epidemiological study, there are limitations and strengths to the present study. Although most previous reports of HPV incidence in EGLs were based on detection from the surface of the lesion, this analysis took advantage of HPV detection within biopsy tissues, which is more likely to represent the viral type present in the lesion.30 Further strengths of this study include the clinical data from a large sample size of men followed for genital disease in Brazil with very high retention rates (>80% at five years of follow-up). Moreover, men were followed-up every six months to allow for early detection of lesions, which were available for histopathological evaluation and HPV genotyping. Nevertheless, our study may be underestimating HPV infection progression to condyloma, which could have occurred during the six months between study visits.
Taken together, approximately 16% of genital HPV-6 and HPV-11 infections progressed to condylomas with the same HPV type while less than 1% of genital HPV-16 infections progressed to an HPV-16-positive PeIN among Brazilian men. In addition, men continue to be at risk of EGLs across all age groups. This study emphasizes the need for vaccine-related immunity in males and for that immunity to be long lasting across the lifespan to ensure a reduction in the overall burden of infection and diseases caused by HPV.
The 4-valent vaccine offers protection against HPVs 6, 11, 16, and 18 has been demonstrated to be efficacious in the reduction of HPV-related diseases in men.16 In our series, HPV vaccine types were detected in 82.3% and 83.3% in condyloma and PeIN cases, respectively. To date, few countries globally have national vaccine programs that include male vaccination, specifically the USA, Austria, Israel, Switzerland, and Australia. As male HPV infection significantly contributes to infection and subsequent cervical disease in women and disease in men, our data support the implementation in Brazil of gender-neutral national immunization policies, crucial to reduce the overall HPV infection burden and ultimately, disease in both women and men.
Ethical approvalEthical approval was given by the University of South Florida (IRB# 102660), Centro de Referência e Treinamento em DST/AIDS, São Paulo, Brazil, and Instituto Nacional de Salud Pública de México.
Funding statementThis work was supported by the Ludwig Institute for Cancer Research; Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) [Grant number 08/57889-1 to LLV]; Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) [Grant number 573799/2008-3 to LLV]. The infrastructure of the HIM Study cohort was supported through a grant from the National Cancer Institute (NCI), National Institutes of Health (NIH) (R01 CA098803 to ARG). Merck & Co. provided financial support for the data analysis to Fundação Faculdade de Medicina da Universidade de São Paulo on behalf of LLV.
Conflicts of interestARG (IISP39582) and SLS (IISP53280) are current recipient of grant funding from Merck, and ARG and LLV are members of the Merck Advisory Board for HPV prophylactic vaccines. RJCS received travel grants from Merck, Sharp & Dohme. No conflicts of interest are declared for any of the remaining authors.
This research was supported in part by research funding from Merck Sharp & Dohme Corp. The opinions expressed in this paper are those of the authors and do not necessarily represent those of Merck Sharp & Dohme Corp.
The authors would like to thank the HIM Study Teams in the USA (Moffitt Cancer Center, Tampa, FL), Brazil (Centro de Referência e Treinamento em DST/AIDS, Fundação Faculdade de Medicina, Instituto do Câncer do Estado de São Paulo, Ludwig Institute for Cancer Research, São Paulo) and Mexico (Instituto Mexicano del Seguro Social, Instituto Nacional de Salud Pública, Cuernavaca).